377945
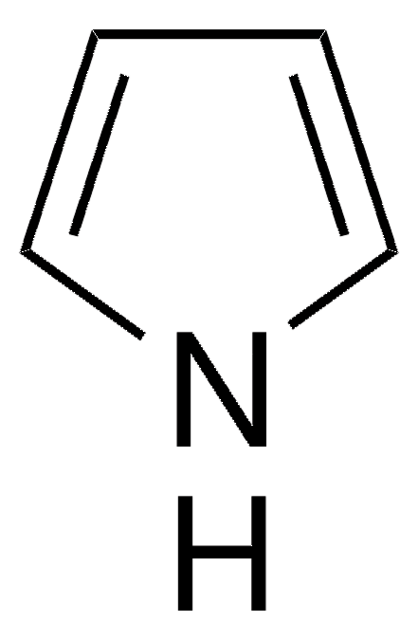
1-(Triisopropylsilyl)pyrrole
95%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C13H25NSi
Numéro CAS:
Poids moléculaire :
223.43
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
95%
Forme
liquid
Indice de réfraction
n20/D 1.492 (lit.)
Point d'ébullition
78 °C/0.4 mmHg (lit.)
Densité
0.904 g/mL at 25 °C (lit.)
Chaîne SMILES
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
Clé InChI
FBQURXLBJJNDBX-UHFFFAOYSA-N
Description générale
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
Application
1-(Triisopropylsilyl)pyrrole may be employed as reagent in perfluoroalkylation and Vilsmeier formylation reactions. It may be used in the preparation of:
- ethyl 2-(2,4-dinitrophenylhydrazono]-3-[ 1-(triisopropylsily1)-pyrrol-2-yflpropanoate
- heterocyclic base, 3-nitropyrrole
- 3-nitropyrrole, required for the synthesis of 1 -(2′-deoxy-β-D-ribofuranosyl)-3-nitropyrrole
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
224.6 °F - closed cup
Point d'éclair (°C)
107 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Daniel A Harki et al.
Biochemistry, 41(29), 9026-9033 (2002-07-18)
Synthetic small molecules that promote viral mutagenesis represent a promising new class of antiviral therapeutics. Ribavirin is a broad-spectrum antiviral nucleoside whose antiviral mechanism against RNA viruses likely reflects the ability of this compound to introduce mutations into the viral
Observation of the cation radicals of pyrrole and of some substituted pyrroles in fast-scan cyclic voltammetry. Standard potentials and lifetimes.
Andrieux CP, et al.
Journal of the American Chemical Society, 112(6), 2439-2440 (1990)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique