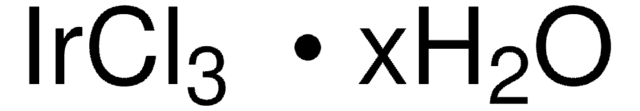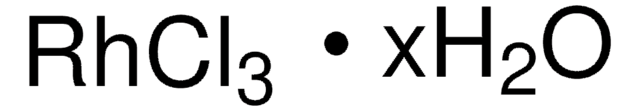206229
Ruthenium(III) chloride hydrate
ReagentPlus®
Synonyme(s) :
Ruthenium trichloride
About This Item
Produits recommandés
Qualité
reagent
Niveau de qualité
Gamme de produits
ReagentPlus®
Forme
powder and chunks
Composition
Degree of hydration, ≤1
Ruthenium content, 40.00-49.00%
Pertinence de la réaction
reagent type: catalyst
core: ruthenium
Impuretés
≤0.1% Insoluble matter (C=1%, 25% HCl)
Chaîne SMILES
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
Clé InChI
BIXNGBXQRRXPLM-UHFFFAOYSA-K
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Caractéristiques et avantages
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Hydrogen is one of the most important resources in providing food, fuel, and chemical products for our everyday life. Sustainable catalytic hydrogen production from bioethanol has gained significant attention in recent years due to globally diminishing fossil fuel supplies, which have necessitated the search for new chemical feedstocks.
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Global Trade Item Number
| Référence | GTIN |
|---|---|
| 206229-1KG | |
| 206229-500G | |
| 206229-1G | 4061835517831 |
| 206229-25G | 4061835521258 |
| 206229-5G | 4061835515387 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique