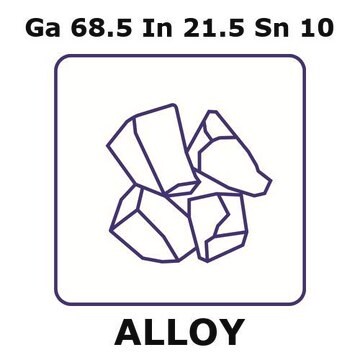
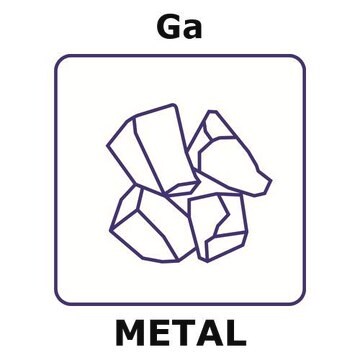
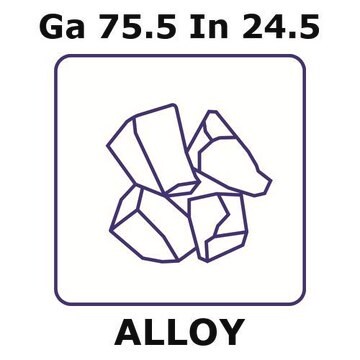
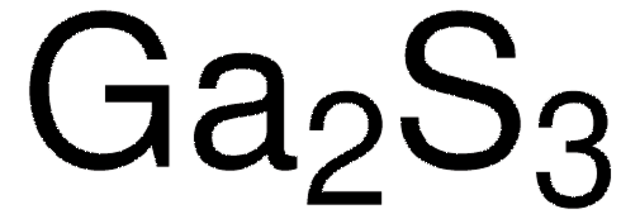203319
Gallium
99.9995% trace metals basis
About This Item
Produits recommandés
Niveau de qualité
Pureté
99.9995% trace metals basis
Forme
(metallic liquid or solid (ambient temp dependent))
Résistivité
25.795 μΩ-cm, 30°C
Point d'ébullition
2403 °C (lit.)
Pf
29.8 °C (lit.)
Densité
5.904 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
[Ga]
InChI
1S/Ga
Clé InChI
GYHNNYVSQQEPJS-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 3 - Met. Corr. 1
Code de la classe de stockage
8B - Non-combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Solid state and materials chemistry have made a tremendous impact and have experienced growth in recent years, particularly for rare earthcontaining materials.
Technologies are an integral part of our lives and we rely on them for such things as communication, heating and cooling, transportation, and construction. Improvements to technologies have made what they do for us more precise, automated, efficient, and powerful.
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
The unique properties of the rare-earth elements and their alloys have brought them from relative obscurity to high profile use in common hightech applications.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique