61148
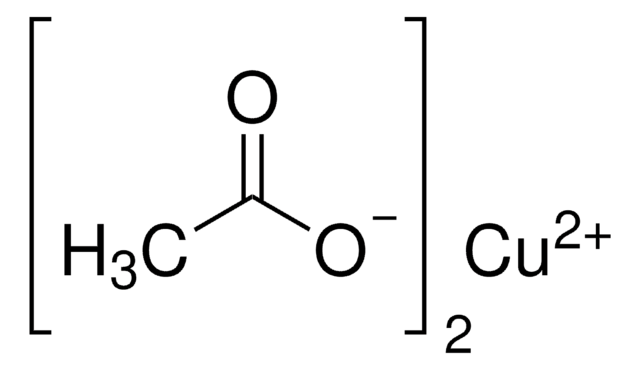
Kupfer(II)-acetat Monohydrat
puriss. p.a., ≥99.0% (RT)
Synonym(e):
Cupric acetate monohydrate
About This Item
Empfohlene Produkte
Dampfdichte
6.8 (vs air)
Qualität
puriss. p.a.
Assay
≥99.0% (RT)
Form
powder or crystals
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Verunreinigungen
≤0.01% total nitrogen (N)
pH-Wert
5.2-5.5 (20 °C, 20 g/L)
Anionenspuren
chloride (Cl-): ≤10 mg/kg
sulfate (SO42-): ≤500 mg/kg
Kationenspuren
Ca: ≤20 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Fe: ≤50 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤20 mg/kg
Zn: ≤50 mg/kg
Grünere Alternativprodukt-Kategorie
SMILES String
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
InChIKey
NWFNSTOSIVLCJA-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Impact of temperature on the physicochemical, structural and biological features of copper-silica nanocomposites.: This study investigates how temperature variations affect the properties of copper-silica nanocomposites. The research focuses on the physicochemical and structural changes that occur and their subsequent impact on biological applications. The findings provide valuable insights for the development of advanced materials with tailored properties for specific applications (Dulski et al., 2020).
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
does not flash
Flammpunkt (°C)
does not flash
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.