229601
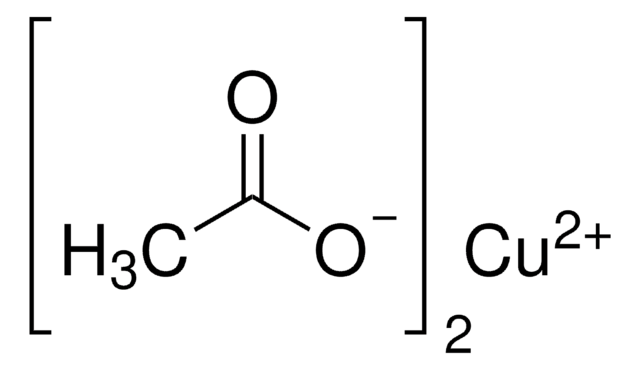
Kupfer(II)-acetat Monohydrat
99.99% trace metals basis
Synonym(e):
Cupric acetate monohydrate
About This Item
Empfohlene Produkte
Dampfdichte
6.8 (vs air)
Assay
99.99% trace metals basis
Form
powder or crystals
Eignung der Reaktion
core: copper
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Grünere Alternativprodukt-Kategorie
SMILES String
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
InChIKey
NWFNSTOSIVLCJA-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
does not flash
Flammpunkt (°C)
does not flash
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
In this article, we will discuss coinage metal deposition processes in order to provide a sense of the most critical precursors, reducing agents, and processes.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.