326755
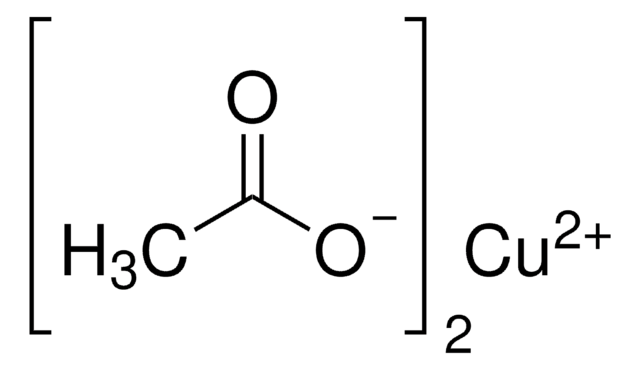
Kupfer(II)-acetat
98%
Synonym(e):
Cupric acetate
About This Item
Empfohlene Produkte
Dampfdichte
6.9 (vs air)
Qualitätsniveau
Assay
98%
Form
powder or crystals
Eignung der Reaktion
reaction type: click chemistry
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Grünere Alternativprodukt-Kategorie
, Aligned
SMILES String
CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChIKey
OPQARKPSCNTWTJ-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Copper(II) acetate also known as cupric acetate, can be used as a catalyst in various processes in the field of greener chemistry. It is particularly useful in cross-coupling reactions, where it can promote the formation of carbon-carbon or carbon-heteroatom bonds, without the need for hazardous reagents or solvents
Anwendung
Copper-catalyzed reductive amination of aromatic and aliphatic ketones with anilines using environmental-friendly molecular hydrogen
Copper(II) acetate is used as a catalyst:
- In the N-arylation of α-amino esters with p-tolylboronic acid to synthesize biaryls via cross-coupling reactions
- In the the synthesis of substituted isoxazole derivatives
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
does not flash
Flammpunkt (°C)
does not flash
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
In this article, we will discuss coinage metal deposition processes in order to provide a sense of the most critical precursors, reducing agents, and processes.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.