45674
Erythromycin
tested according to Ph. Eur.
Synonym(e):
Erythromycinum
About This Item
Empfohlene Produkte
Agentur
EPA 1694
USP/NF
tested according to Ph. Eur.
Qualitätsniveau
Form
solid
Farbe
white to faint yellow
Löslichkeit
H2O: soluble 2 mg/mL
acetone: freely soluble
acetonitrile: freely soluble
alcohol: soluble
amyl acetate: moderately soluble
chloroform: soluble
diethyl ether: soluble
ethyl acetate: freely soluble
Wirkungsspektrum von Antibiotika
Gram-negative bacteria
Gram-positive bacteria
Anwendung(en)
environmental
Wirkungsweise
protein synthesis | interferes
SMILES String
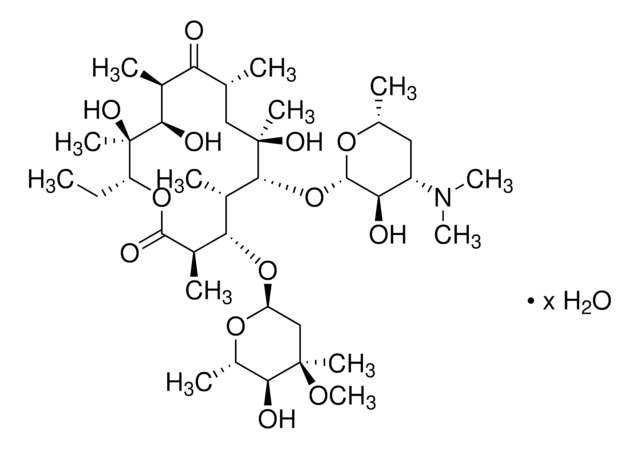
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChIKey
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Angaben zum Gen
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Antimikrobielles Spektrum: Gram-negative und Gram-positive Bakterien.
Verpackung
Vorsicht
Angaben zur Herstellung
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.