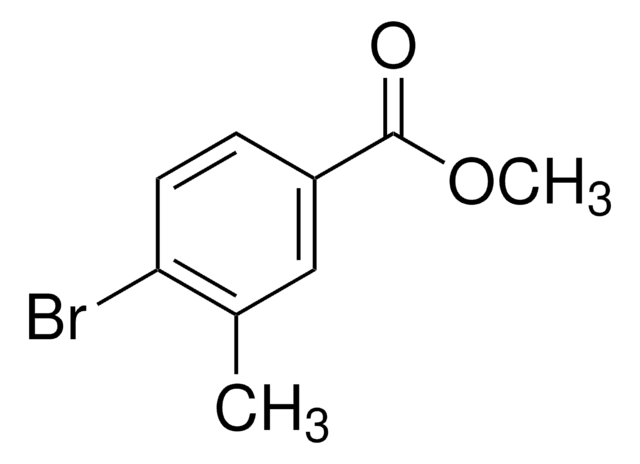
L511188
(S)-2-(2,3-Bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride
AldrichCPR
Synonym(e):
(βS)-β-[[2,3-bis(dicyclohexylamino)-2-cyclopropen-1-ylidene]amino]-benzenepropanol hydrochloride (1:1), Dicyclohexyl cyclopropenimine, Lambert Cyclopropenimine Catalyst
About This Item
Empfohlene Produkte
SMILES String
Cl.OC[C@H](Cc1ccccc1)\N=C2\C(N(C3CCCCC3)C4CCCCC4)=C2N(C5CCCCC5)C6CCCCC6
InChI
1S/C36H55N3O.ClH/c40-27-29(26-28-16-6-1-7-17-28)37-34-35(38(30-18-8-2-9-19-30)31-20-10-3-11-21-31)36(34)39(32-22-12-4-13-23-32)33-24-14-5-15-25-33;/h1,6-7,16-17,29-33,40H,2-5,8-15,18-27H2;1H/t29-;/m0./s1
InChIKey
RTCSAEOYHXMTHG-JMAPEOGHSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

Sonstige Hinweise
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.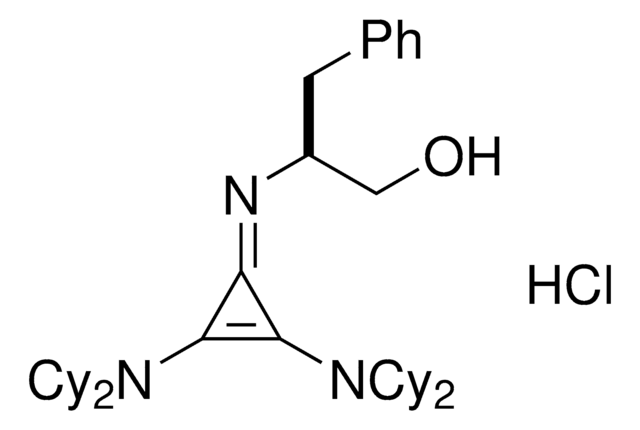

![3-{[(1R,2R)-2-(1-Piperidinyl)-cyclohexyl]-amino}-4-{[4-(trifluormethyl)-phenyl]-amino}-3-cyclobuten-1,2-dion 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)