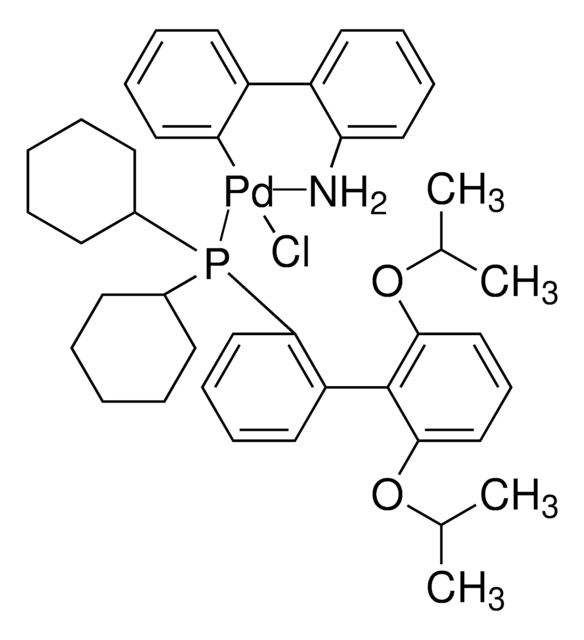
763004
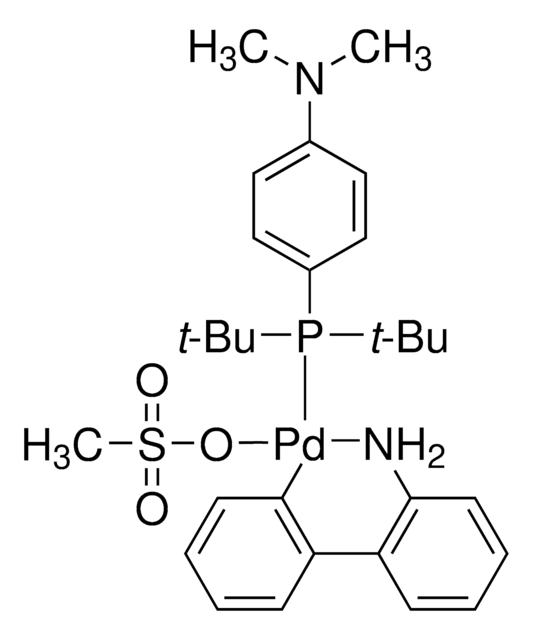
CPhos Pd G3
95%
Synonym(e):
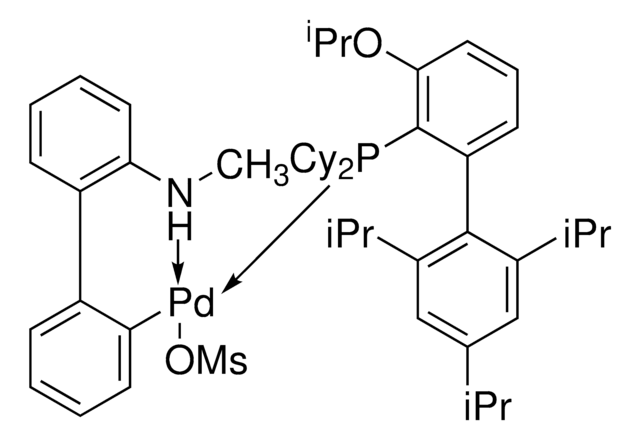
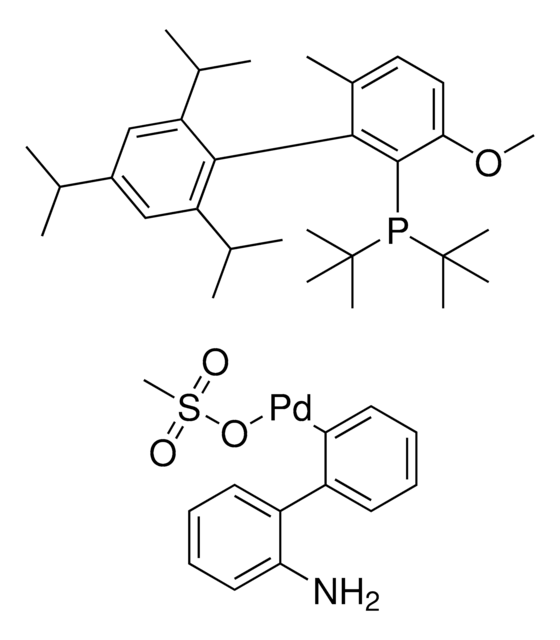
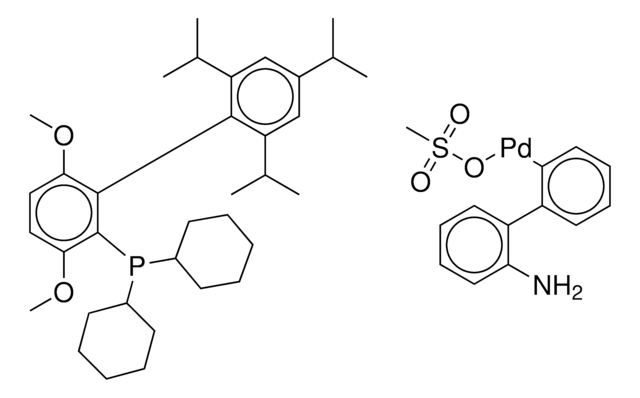
CPhos-G3-Palladacycle, CPhos-Pd-G3, [(2-Dicyclohexylphosphino-2′,6′-bis(N,N-dimethylamino) -1,1′-biphenyl)-2-(2′-amino-1,1′-biphenyl)] palladium(II) methanesulfonate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
solid
Leistungsmerkmale
generation 3
Eignung der Reaktion
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp (Schmelzpunkt)
176-178 °C (decomposition)
Funktionelle Gruppe
phosphine
SMILES String
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CN(C)c3cccc(N(C)C)c3-c4ccccc4P(C5CCCCC5)C6CCCCC6
InChI
1S/C28H41N2P.C12H10N.CH4O3S.Pd/c1-29(2)25-19-13-20-26(30(3)4)28(25)24-18-11-12-21-27(24)31(22-14-7-5-8-15-22)23-16-9-6-10-17-23;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h11-13,18-23H,5-10,14-17H2,1-4H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChIKey
SMFSVINURNWOPR-UHFFFAOYSA-M
Allgemeine Beschreibung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphin)ferrocen]dichlorpalladium(II), Komplex mit Dichlormethan](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)