Wichtige Dokumente
488216
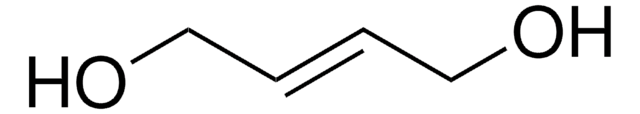
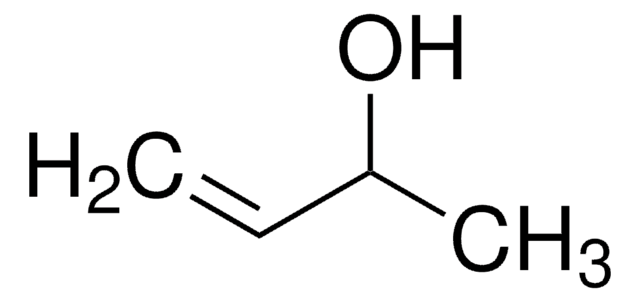
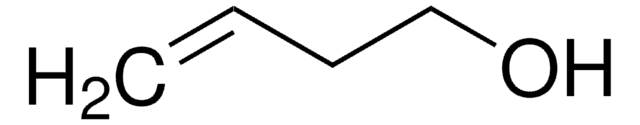
3,4-Dihydroxy-1-buten
≥99%
Synonym(e):
3-Buten-1,2-diol
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99%
bp
195 °C/733 mmHg (lit.)
Dichte
1.047 g/mL at 25 °C (lit.)
Funktionelle Gruppe
hydroxyl
SMILES String
OCC(O)C=C
InChI
1S/C4H8O2/c1-2-4(6)3-5/h2,4-6H,1,3H2
InChIKey
ITMIAZBRRZANGB-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
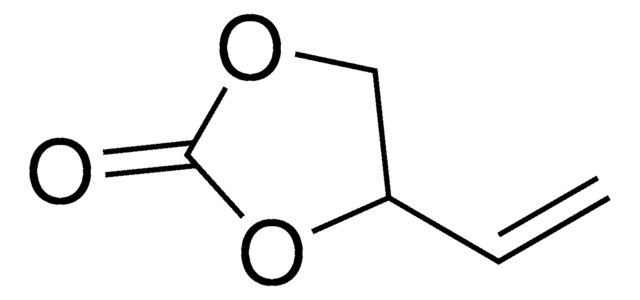
- As a reactant to synthesize cyclic organic carbonates by continuous flow procedure.
- To prepare substituted oxazolidinone ligands used to target medicinally relevant RNAs.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.