Alle Fotos(1)
Wichtige Dokumente
479403
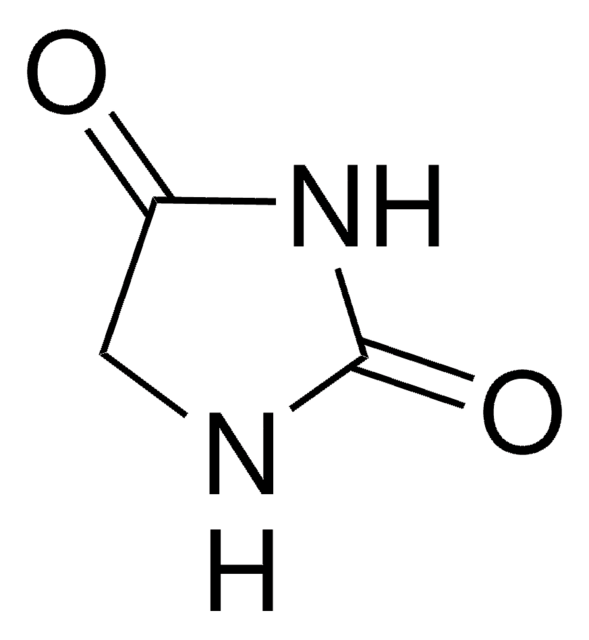
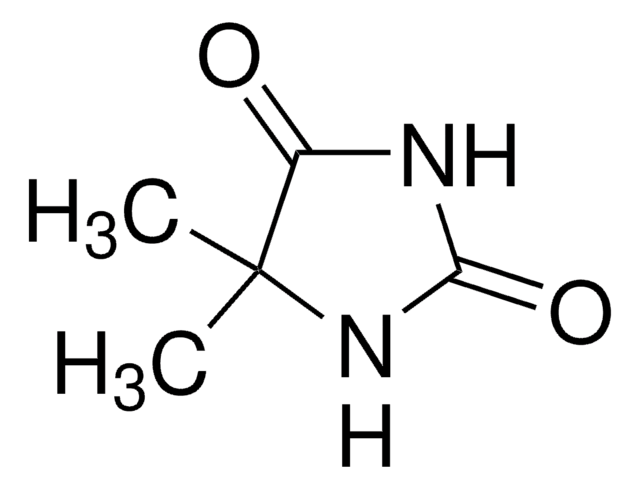
1,5,5-Trimethylhydantoin
98%
Synonym(e):
1,5,5-Trimethyl-2,4-imidazolidinedione, 3,4,4-Trimethyl-2,5-dioxoimidazolidine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H10N2O2
CAS-Nummer:
Molekulargewicht:
142.16
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
161-164 °C (lit.)
SMILES String
CN1C(=O)NC(=O)C1(C)C
InChI
1S/C6H10N2O2/c1-6(2)4(9)7-5(10)8(6)3/h1-3H3,(H,7,9,10)
InChIKey
ZNYIPTYJBRGSSL-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
1,5,5-Trimethylhydantoin (TMH) is a 1,5,5-trisubstituted hydantoin. Its mass spectrum has been recorded and analyzed. The density of TMH is 1.1318g/ml at 25°C.
Anwendung
1,5,5-Trimethylhydantoin (1,5,5-Trimethyl-imidazolidine-2,4-dione) may be used to synthesize 3-bromomethyl-1,5,5-trimethylimidazolid-ine-2,4-dione.
Reactant for:
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Mass spectrometric analysis and theoretical calculations of the occurrence of tautomeric structures of hydantoins.
Allegretti PE, et al.
Afinidad, 57(485), 41-49 (2000)
Use of the cascade α-oxo-amidoalkylation/transposition/Π-cationic cyclization of N-acyliminium ions in the synthesis of novel fused heterocyclic N,O-acetals.
Pesquet A, et al.
ARKIVOC (Gainesville, FL, United States), 8, 27-40 (2010)
Yaws CL.
Thermophysical Properties of Chemicals and Hydrocarbons, 280-280 (2008)
Zi-Ao Huang et al.
Electrophoresis, 41(3-4), 183-193 (2019-12-19)
In this paper, the development of a simple dilute-and-shoot method for quantifying urinary creatinine by CE-ESI-MS was described. The creatinine analysis time was about 7 min/sample by conventional single injection (SI) method and can be significantly reduced to less than 2 min/sample
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.