Alle Fotos(1)
Wichtige Dokumente
357774
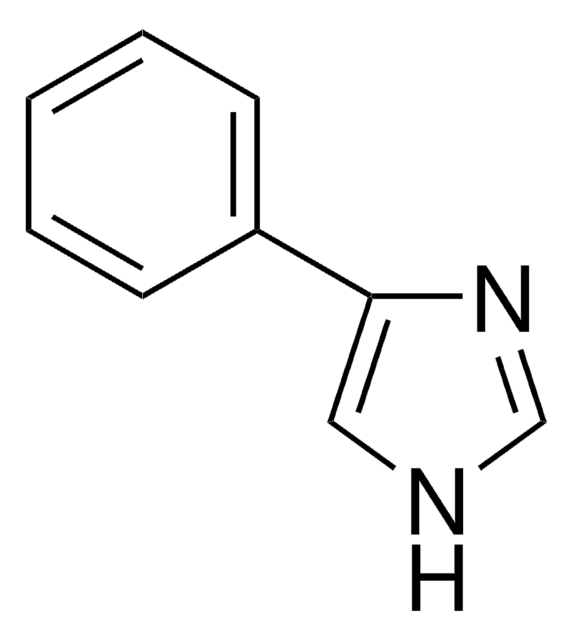
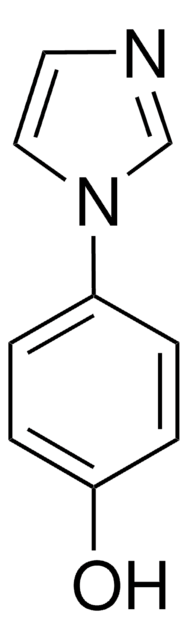
1-Phenylimidazol
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H8N2
CAS-Nummer:
Molekulargewicht:
144.17
MDL-Nummer:
UNSPSC-Code:
12352005
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
liquid
bp
142 °C/15 mmHg (lit.)
mp (Schmelzpunkt)
13 °C (lit.)
Dichte
1.14 g/mL at 25 °C (lit.)
SMILES String
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
InChIKey
SEULWJSKCVACTH-UHFFFAOYSA-N
Allgemeine Beschreibung
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Anwendung
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Priyadarshini Balaraman et al.
Biochimica et biophysica acta. General subjects, 1863(2), 304-312 (2018-11-06)
The camphor-degrading microorganism, Pseudomonas putida strain ATCC 17453, is an aerobic, gram-negative soil bacterium that uses camphor as its sole carbon and energy source. The genes responsible for the catabolic degradation of camphor are encoded on the extra-chromosomal CAM plasmid.
Active-site structure analysis of recombinant human inducible nitric oxide synthase using imidazole.
R M Chabin et al.
Biochemistry, 35(29), 9567-9575 (1996-07-23)
Nitric oxide synthase catalyzes the pyridine nucleotide-dependent oxidation of L-arginine to nitric oxide and L-citrulline. It is a specialized cytochrome P450 monooxygenase that is sensitive to inhibition by imidazole. Steady-state kinetic studies on recombinant human inducible nitric oxide synthase (rH-iNOS)
D M Grant et al.
Biochemical pharmacology, 36(8), 1251-1260 (1987-04-15)
The nature of the cytochrome P-450-dependent enzyme reactions giving rise to four primary metabolites of caffeine was investigated using microsomes isolated from livers of human kidney donors. Metabolite formation proceeded at a lower rate than that predicted from in vivo
P R Kerklaan et al.
Journal of cancer research and clinical oncology, 111(3), 196-202 (1986-01-01)
The effect of the mixed-function oxidase inhibitor phenylimidazole (PI) and the amine oxidase inhibitors iproniazid (IPRO) and aminoacetonitrile (AAN) on the mutagenic activity of various carcinogens was determined in intrasanguineous host-mediated assays, using mice as hosts and E. coli 343/113
P Ammann et al.
Toxicology and applied pharmacology, 149(2), 217-225 (1998-05-08)
Chloroform is carcinogenic in rodents but is not mutagenic or DNA reactive. Chloroform-induced hepatocarcinogenesis in rodents is believed to be secondary to events associated with cytotoxicity and cell proliferation. Understanding the mechanisms of chloroform toxicity may provide insights into the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.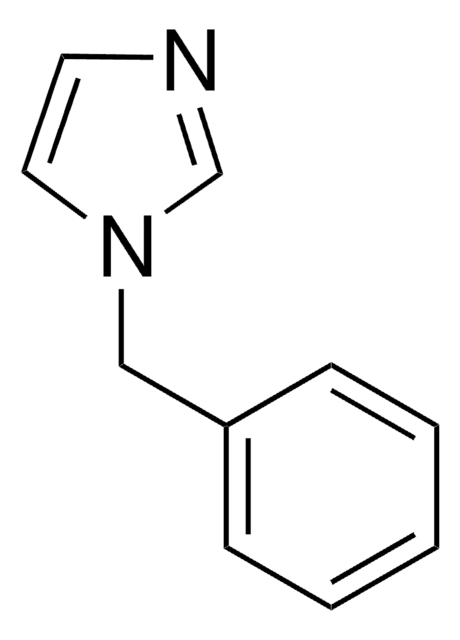



![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)