Wichtige Dokumente
254274
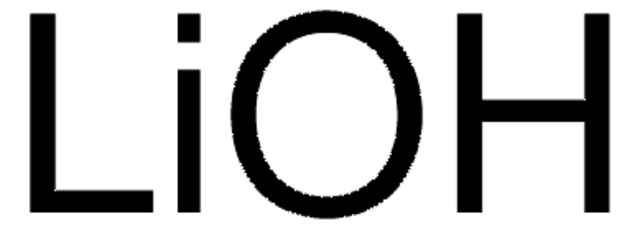
Lithiumhydroxid Monohydrat
99.95% trace metals basis
Synonym(e):
Lithine hydrate
About This Item
Empfohlene Produkte
Qualität
for analytical purposes
Qualitätsniveau
Assay
99.95% trace metals basis
Form
crystalline
Grünere Alternativprodukt-Eigenschaften
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Verunreinigungen
≤550.0 ppm Trace Metal Analysis
Anwendung(en)
battery manufacturing
Grünere Alternativprodukt-Kategorie
SMILES String
[Li+].O.[OH-]
InChI
1S/Li.2H2O/h;2*1H2/q+1;;/p-1
InChIKey
GLXDVVHUTZTUQK-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- To synthesize Olivine LiFePO4 nanoplates with different crystal orientations by glycol-based solvothermal process. It demonstrated that the crystal orientation of cathode nanoplates plays an important role in the performance.
- As a precursor for the synthesis of LiFePO4 platelets under hydrothermal synthesis conditions. It exhibited homogeneous particle size distribution, greater electrochemical performances and cycle life, and morphology control.
- To synthesize Ni-rich Li[NixCoyMn1–x–y]O2 gradient cathodes (NCM). These cathodes exhibited enhanced cycling and chemical stability due to their strong crystallographic texture, unique particle morphology, and highly correlated particle orientation.
- As a precursor to prepare porous salt hydrate-based composites for low-temperature thermochemical heat storage.
- As an electrolyte additive in lithium-ion batteries.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 254274-50G | 4061838138668 |
| 254274-10G | 4061838138651 |
| 254274-250G | 4061838252715 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.