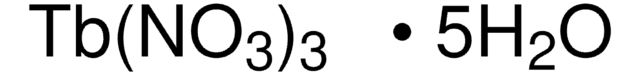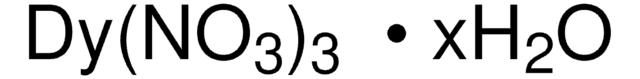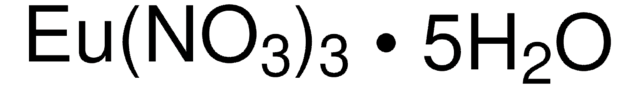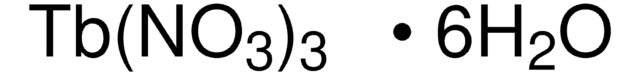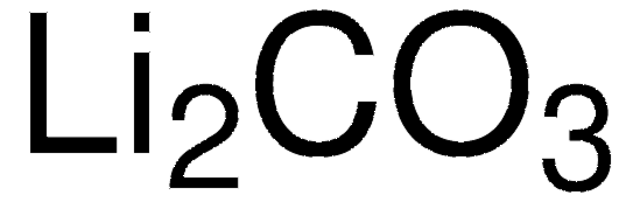229733
Lithiumbromid
powder and chunks, ≥99.995% trace metals basis
Synonym(e):
Lithium monobromide
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99.995% trace metals basis
Form
powder and chunks
Verunreinigungen
≤50.0 ppm Trace Metal Analysis
mp (Schmelzpunkt)
550 °C (lit.)
Anwendung(en)
battery manufacturing
SMILES String
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
InChIKey
AMXOYNBUYSYVKV-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Space cooling using geothermal single‐effect water/lithium bromide absorption chiller: This research explores the use of lithium bromide in geothermal absorption chillers for space cooling applications (M El Haj Assad, M Sadeghzadeh, 2021).
- A facile and fast method for quantitating lignin in lignocellulosic biomass using acidic lithium bromide trihydrate (ALBTH): The paper introduces a novel method for lignin quantification using lithium bromide trihydrate (N Li, X Pan, J Alexander, 2016).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.