Alle Fotos(2)
Wichtige Dokumente
157872
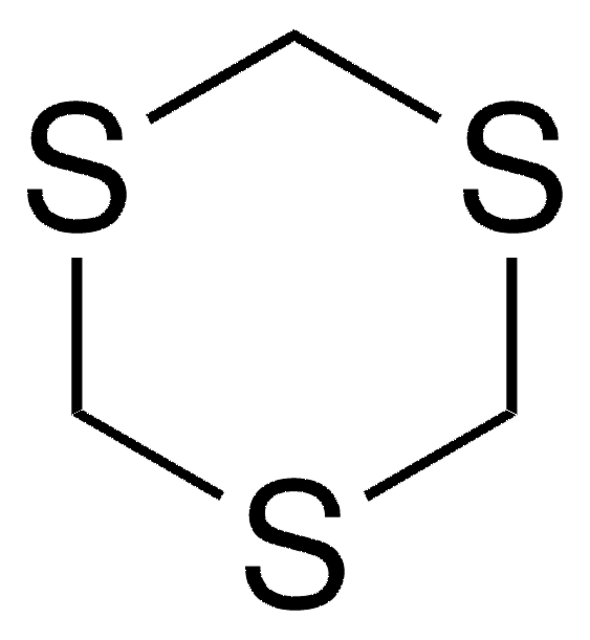
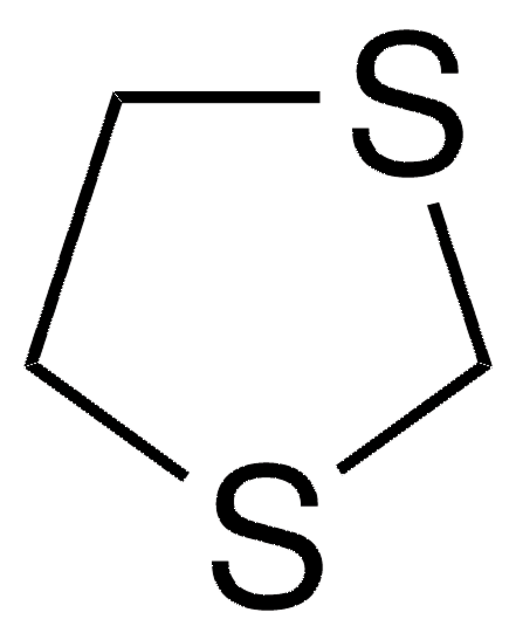
1,3-Dithian
97%
Synonym(e):
m-Dithiane (7CI), m-Dithiane (8CI)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C4H8S2
CAS-Nummer:
Molekulargewicht:
120.24
Beilstein:
102534
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
solid
mp (Schmelzpunkt)
52-54 °C (lit.)
Funktionelle Gruppe
thioether
SMILES String
C1CSCSC1
InChI
1S/C4H8S2/c1-2-5-4-6-3-1/h1-4H2
InChIKey
WQADWIOXOXRPLN-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
1,3-Dithiane, a protected formaldehyde anion equivalent, serves as useful labeled synthon.
Anwendung
1,3-Dithiane was used as reagent for deoxygenation of sulfoxides to their corresponding sulfides.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
194.0 °F - closed cup
Flammpunkt (°C)
90 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
A I Noskov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(1), 6-10 (2010-07-17)
The IR spectra of 1,3-dithiane-1-oxide (I) and 1,3-dithia-1-oxocyclohept-5-ene (II) were recorded in solution, solid and liquid phase over 4000-400 cm(-1) spectral range. It was found that both (I) and (II) in liquid phase and solutions exist in two conformations: (I)
Yuncong Chen et al.
Chemical communications (Cambridge, England), 48(42), 5094-5096 (2012-04-20)
A novel sensitive and specific Hg(2+) chemodosimeter, derived from 1',3'-dithiane-substituted 2,1,3-benzoxadiazole, displays "turn-on" fluorescent and colorimetric responses via an Hg(2+)-triggered aldehyde recovery reaction. Its potential to monitor Hg(2+) in living organisms has been demonstrated using zebrafish larvae.
Nasser Iranpoor et al.
The Journal of organic chemistry, 67(9), 2826-2830 (2002-04-27)
A new, mild, and novel method is described for the efficient deoxygenation of sulfoxides to their corresponding sulfides with 1,3-dithiane at room temperature in the presence of catalytic amounts of N-bromosuccinimide (NBS), 2,4,4,6-tetrabromo-2,5-cyclohexadienone (TABCO), or Br(2) as the source of
Al-Monsur Jiaul Haque et al.
Chemical communications (Cambridge, England), (32)(32), 4865-4867 (2009-08-05)
We report the use of 1,3-dithiane combined with aryldiazonium cation for the immobilization of biomolecules based on electrochemical addressing.
Valerie J Peterson et al.
The Biochemical journal, 362(Pt 1), 173-181 (2002-02-07)
Apo and holo forms of retinoic acid receptors, and other nuclear receptors, display differential sensitivity to proteolytic digestion that likely reflects the distinct conformational states of the free and liganded forms of the receptor. We have developed a method for
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.