33586
Flunixin
VETRANAL®, analytical standard
Synonyme(s) :
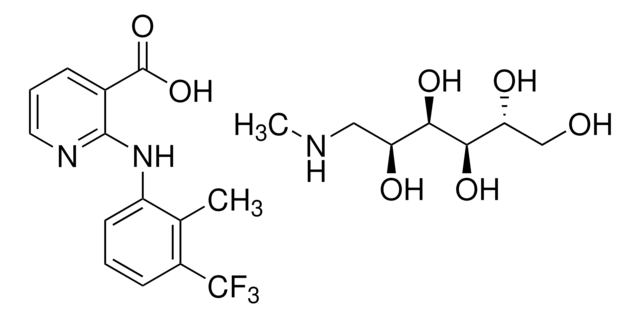
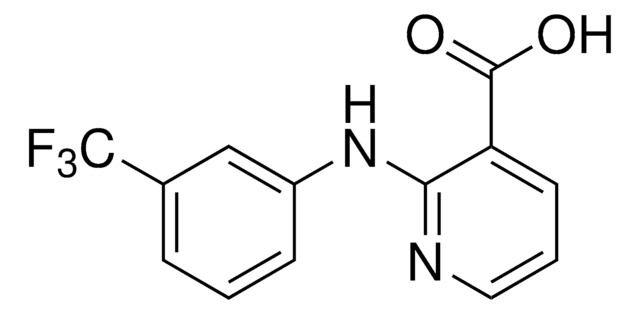
2-[2-Methyl-3-(trifluoromethyl)phenylamino]nicotinic acid
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Gamme de produits
VETRANAL®
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
forensics and toxicology
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
Cc1c(Nc2ncccc2C(O)=O)cccc1C(F)(F)F
InChI
1S/C14H11F3N2O2/c1-8-10(14(15,16)17)5-2-6-11(8)19-12-9(13(20)21)4-3-7-18-12/h2-7H,1H3,(H,18,19)(H,20,21)
Clé InChI
NOOCSNJCXJYGPE-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Bovine muscle samples by hydrophilic interaction liquid chromatography-electrospray-tandem mass spectrometry (HILIC-ESI-MS/MS) equipped with selected reaction monitoring (SRM) detection.
- Animal tissues by dispersive-solid phase extraction (d-SPE) and enhanced matrix removal for lipids (EMR-L), followed by analysis using ultra-high performance liquid chromatography-triple quadrupole or quadrupole-time-of-flight (UHPLC-QqQ or UHPLC-Q/TOF) methods in conjunction with ESI-MS/MS operating on multiple reaction monitoring (MRM) mode of detection as well as ESI-LC-MS/MS with SRM detection mode.
- Porcine muscle samples by ESI-LC-MS/MS with MRM detection mode.
Produits recommandés
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique