244988
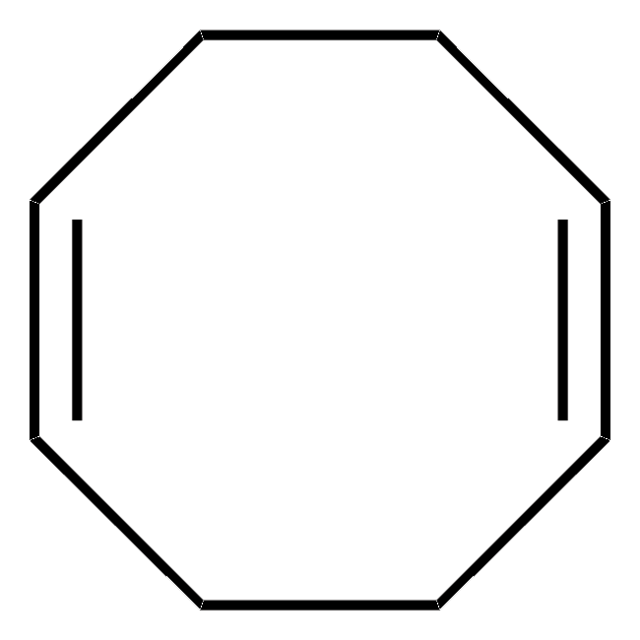
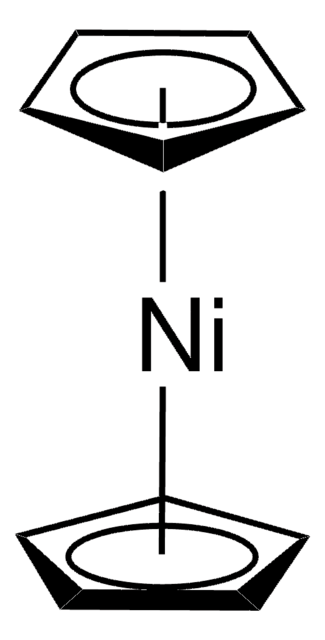
Bis(1,5-cyclooctadiene)nickel(0)
Synonyme(s) :
Bis(cyclooctadiene)nickel, Ni(COD)2
About This Item
Produits recommandés
Niveau de qualité
Pertinence de la réaction
core: nickel
reagent type: catalyst
Capacité de réaction
reaction type: Cross Couplings
Paramètres
temperature sensitive
Pf
60 °C (dec.) (lit.)
Température de stockage
−20°C
Chaîne SMILES
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
Clé InChI
JRTIUDXYIUKIIE-KZUMESAESA-N
Application
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
Organes cibles
Lungs
Code de la classe de stockage
4.1B - Flammable solid hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Csp2- and Csp-hybridized coupling reactions are established catalytic approaches. However, multi-step Csp3- and Csp2-coupling reactions of boronic acids and related derivatives are still limited by ineffective two-electron transmetalation reactions.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique