F3055
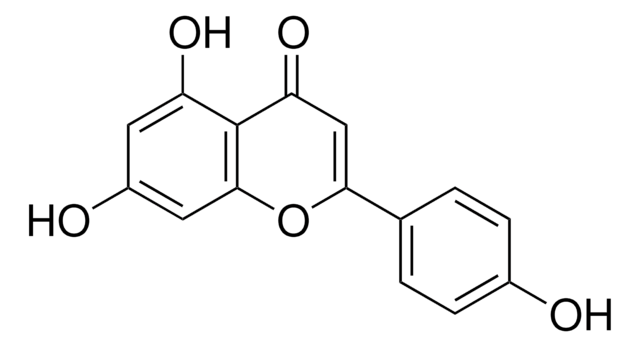
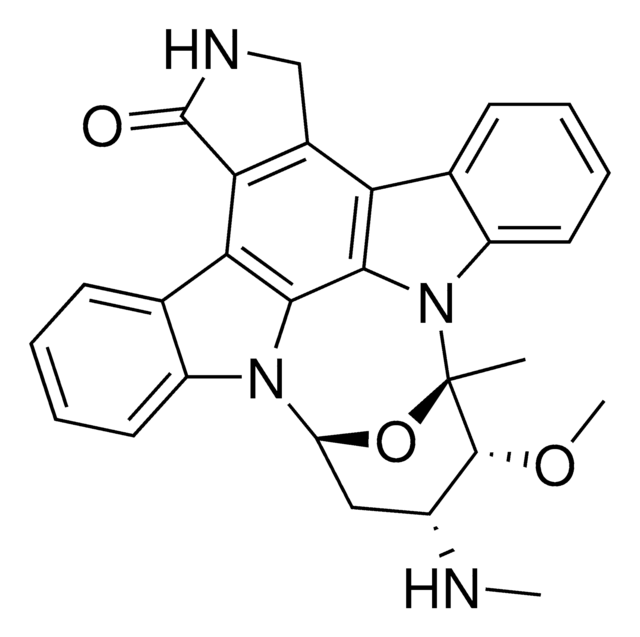
Flavopiridol hydrochloride hydrate
≥98% (HPLC), powder
Synonym(s):
(−)-2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-4H-1-benzopyran-4-one hydrochloride, L-86-8276, NSC-649890
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
color
white to light brown
solubility
H2O: ~2 mg/mL
DMSO: >5 mg/mL
storage temp.
2-8°C
SMILES string
OC1=C(C(C=C(C2=C(Cl)C=CC=C2)O3)=O)C3=C([C@H]4CCN(C)C[C@H]4O)C(O)=C1.Cl
InChI
1S/C21H20ClNO5.ClH/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22;/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3;1H/t12-,17+;/m0./s1
InChI key
LGMSNQNWOCSPIK-LWHGMNCYSA-N
Gene Information
human ... CDK1(983) , CDK2(1017) , CDK4(1019) , CDK6(1021) , CDK7(1022) , CDK9(1025)
Application
- as a cyclin-dependent kinase 9 (CDK9) inhibitor to study its effects on histone H3 methylation at lysine 36 (H3K36) and deactivation of transcription in porcine fetal fibroblasts
- as an RNA polymerase inhibitor to study its effects on hepatic cells
- as RNA transcription inhibitor to study its effects on euchromatin coarsening in zebrafish embryo
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Human epithelial intestinal colonic organoids can be used as an alternative to Caco-2 drug permeability assays for drug screening and compound toxicity testing.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service