SRP5191
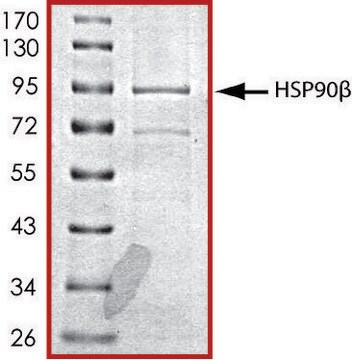
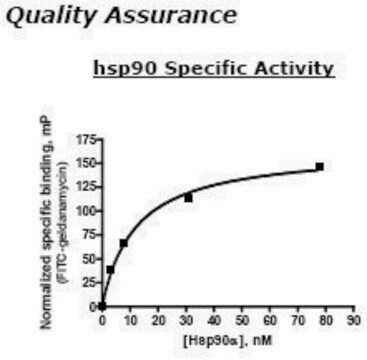
HSP90 α, His tagged human
recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
Sinônimo(s):
FLJ31884, HSP86, HSP90AA1, HSP90N, HSPC1, HSPCA, HSPCAL1, HSPCAL4, HSPN, Hsp89, Hsp90, LAP2
About This Item
Produtos recomendados
fonte biológica
human
recombinante
expressed in baculovirus infected Sf9 cells
Ensaio
≥70% (SDS-PAGE)
Formulário
buffered aqueous glycerol solution
peso molecular
~94 kDa
técnica(s)
cell culture | mammalian: suitable
solubilidade
water: soluble
adequação
suitable for molecular biology
nº de adesão NCBI
Condições de expedição
dry ice
temperatura de armazenamento
−70°C
Informações sobre genes
human ... HSP90AA1(3320)
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
forma física
Nota de preparo
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica