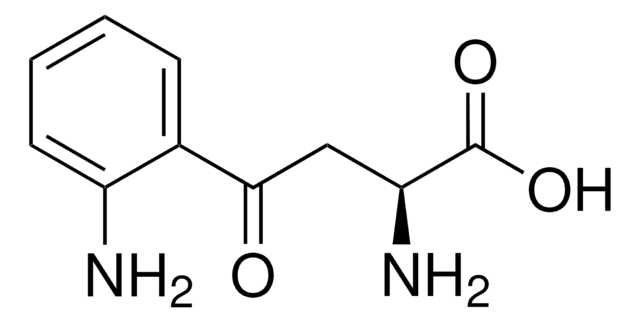
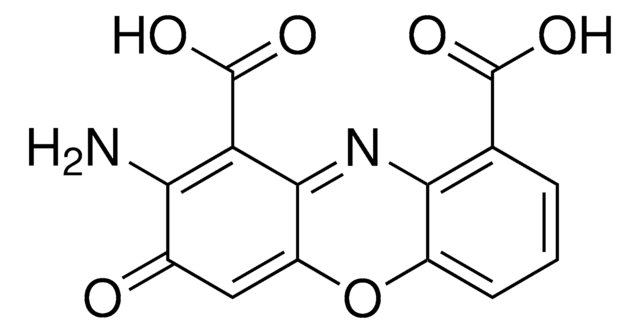
SML0096
Cinnabarinic Acid
≥98% (HPLC)
Sinônimo(s):
2-amino-3-oxo-3H-phenoxazine-1,9-dicarboxylic acid, Cinnabaric acid
About This Item
Produtos recomendados
Ensaio
≥98% (HPLC)
Formulário
powder
condição de armazenamento
desiccated
cor
red to very dark red
solubilidade
DMSO: ≥4 mg/mL
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
NC1=C(C(O)=O)C2=Nc3c(OC2=CC1=O)cccc3C(O)=O
InChI
1S/C14H8N2O6/c15-10-6(17)4-8-12(9(10)14(20)21)16-11-5(13(18)19)2-1-3-7(11)22-8/h1-4H,15H2,(H,18,19)(H,20,21)
chave InChI
FSBKJYLVDRVPTK-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
Ações bioquímicas/fisiológicas
Cinnabarinic acid (CA) connects between initiation of the kynurenine pathway and immune tolerance that is used to prevent neuroinflammation.
Características e benefícios
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Conteúdo relacionado
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica