About This Item
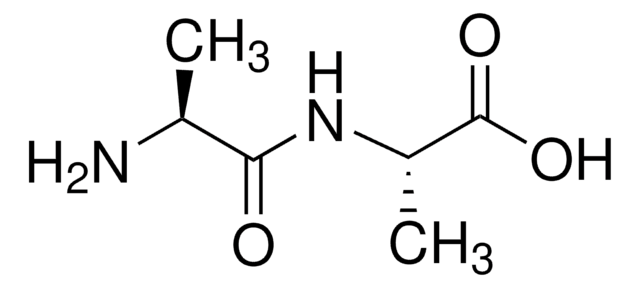
Fórmula empírica (Notação de Hill):
C8H12N4O3
Número CAS:
Peso molecular:
212.21
Número MDL:
Código UNSPSC:
12352202
ID de substância PubChem:
NACRES:
NA.26
Produtos recomendados
Nome do produto
Gly-His,
Formulário
powder
Nível de qualidade
cor
white
temperatura de armazenamento
−20°C
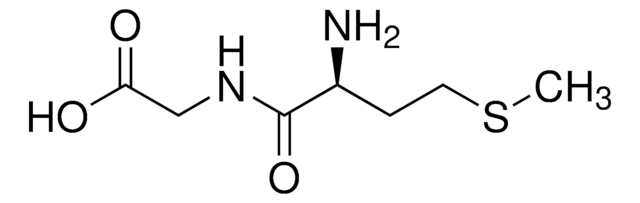
cadeia de caracteres SMILES
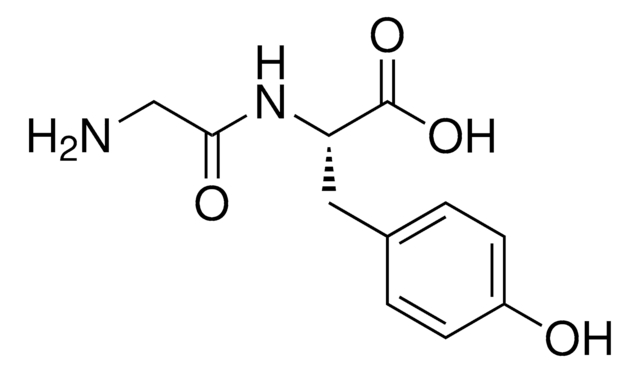
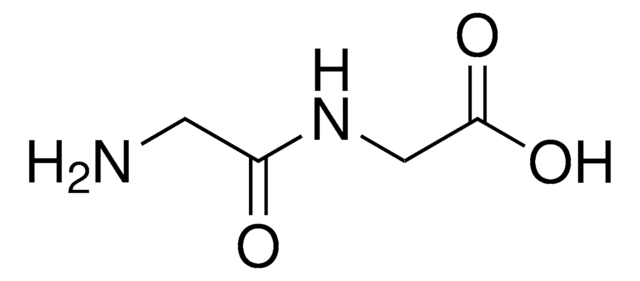
NCC(=O)NC(Cc1cnc[nH]1)C(O)=O
InChI
1S/C8H12N4O3/c9-2-7(13)12-6(8(14)15)1-5-3-10-4-11-5/h3-4,6H,1-2,9H2,(H,10,11)(H,12,13)(H,14,15)
chave InChI
YIWFXZNIBQBFHR-UHFFFAOYSA-N
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Giulio Vistoli et al.
Biochemical and biophysical research communications, 492(3), 487-492 (2017-08-24)
The study combines HPLC-based with MS-based competitive analyses to evaluate the quenching activity of a set of carnosine derivatives towards methylglyoxal (MGO) and malondialdehyde (MDA) chosen as representative of α- and β-dicarbonyls, respectively. The obtained results underline that these derivatives
Girolamo Casella et al.
Inorganic chemistry, 47(11), 4796-4807 (2008-05-08)
We have tested several computational protocols, at the nonrelativistic DFT level of theory, for the calculation of 1J(119Sn, 13C) and 2J(119Sn, 1H) spin-spin coupling constants in di- and trimethyltin(IV) derivatives with various ligands. Quite a good agreement with experimental data
E Matczak-Jon et al.
Journal of inorganic biochemistry, 12(2), 143-156 (1980-04-01)
1H and 13C nmr studies on the Pd(II)Gly-His complex interaction with cytidine and GMP have shown that the nucleoside binds the palladium complex via N3 nitrogen and the nucleotide binds that complex via N7 nitrogen. The analysis of the Cyd
Giampiero De Sanctis et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 11(2), 153-167 (2005-12-13)
The pH dependence of redox properties, spectroscopic features and CO binding kinetics for the chelated protohemin-6(7)-L-histidine methyl ester (heme-H) and the chelated protohemin-6(7)-glycyl-L-histidine methyl ester (heme-GH) systems has been investigated between pH 2.0 and 12.0. The two heme systems appear
Flora Carrera et al.
Inorganic chemistry, 43(21), 6674-6683 (2004-10-13)
Knowledge of the complexes formed by N-coordinating ligands and Cu(II) ions is of relevance in understanding the interactions of this ion with biomolecules. Within this framework, we investigated Cu(II) complexation with mono- and polydentate ligands, such as ammonia, ethylenediamine (en)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica