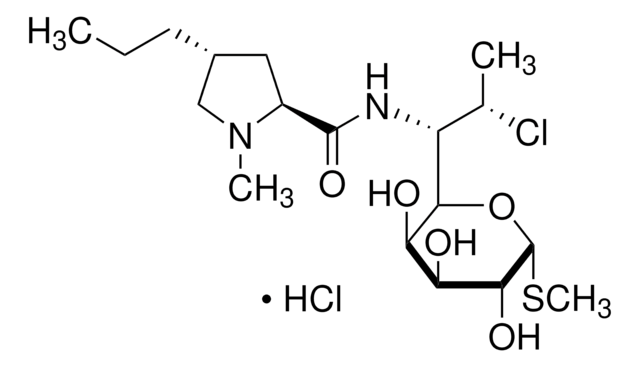
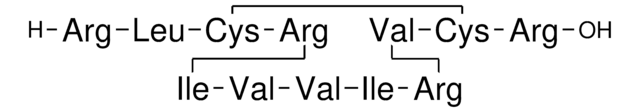C5241
Cinnamycin
from Streptomyces cinnamoneus, ≥95% (HPLC)
Sinônimo(s):
Lanthiopeptin, NSC-71936, Ro 09-0198
About This Item
Produtos recomendados
fonte biológica
Streptomyces cinnamoneus
Nível de qualidade
Ensaio
≥95% (HPLC)
forma
solid
solubilidade
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
espectro de atividade do antibiótico
fungi
Modo de ação
cell membrane | interferes
temperatura de armazenamento
2-8°C
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
chave InChI
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Categorias relacionadas
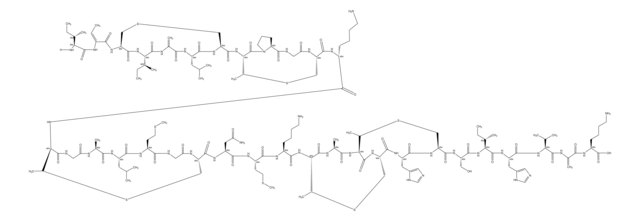
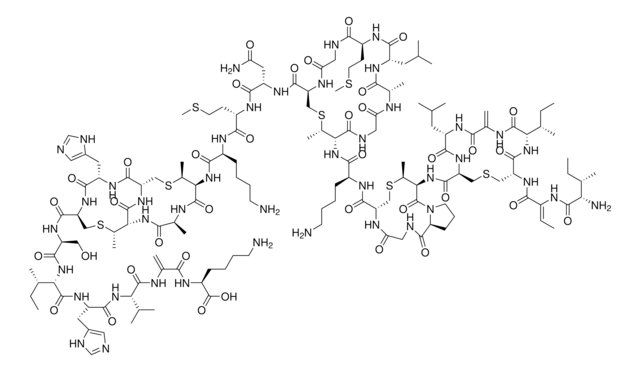
Amino Acid Sequence
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
With bacterial resistance and emerging infectious diseases becoming potential threats to humans, ribosomally synthesized antimicrobial peptides have become a promising focus area in antibiotic research.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica