D0740000
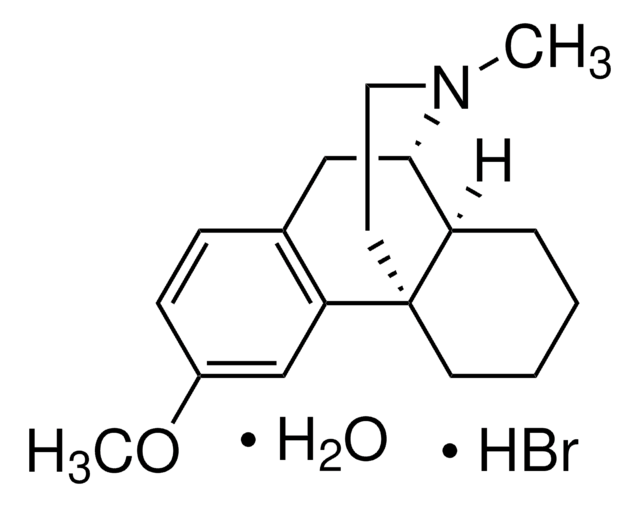
Dextromethorphan hydrobromide
European Pharmacopoeia (EP) Reference Standard
Sinônimo(s):
Dextromethorphan hydrobromide monohydrate, (9S,13S,14S)-3-Methoxy-17-methylmorphinan hydrobromide
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
dextromethorphan
fabricante/nome comercial
EDQM
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
aplicação(ões)
pharmaceutical (small molecule)
Formato
neat
cadeia de caracteres SMILES
Br[H].[H]O[H].[H][C@]12CCCC[C@]13CCN(C)[C@H]2Cc4ccc(OC)cc34
InChI
1S/C18H25NO.BrH.H2O/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18;;/h6-7,12,15,17H,3-5,8-11H2,1-2H3;1H;1H2/t15-,17+,18+;;/m1../s1
chave InChI
STTADZBLEUMJRG-IKNOHUQMSA-N
Informações sobre genes
human ... GRIN2A(2903) , SIGMAR1(10280)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Embalagem
Outras notas
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Aquatic Chronic 2
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica