01580590
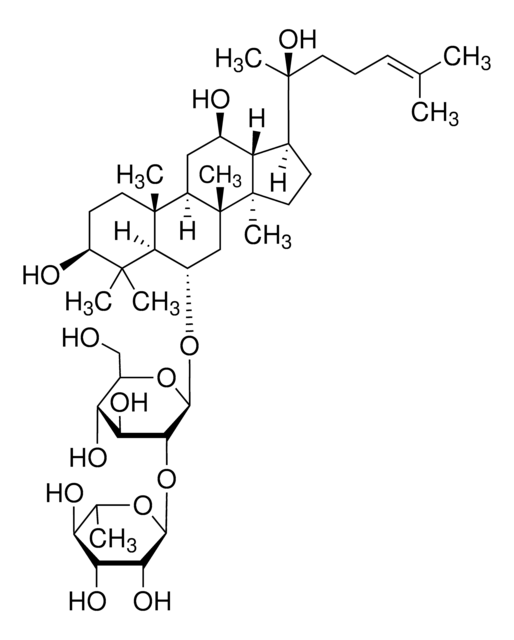
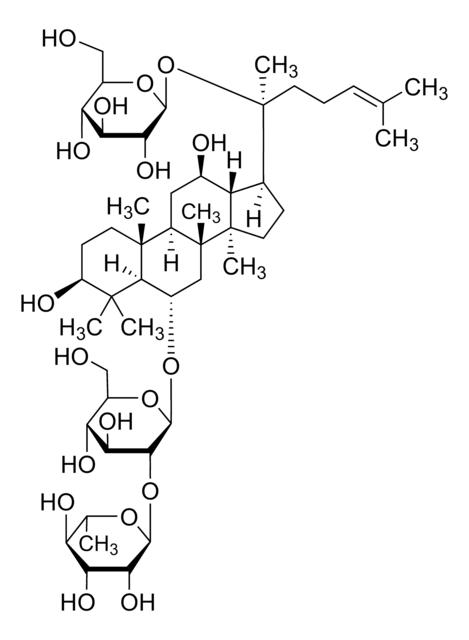
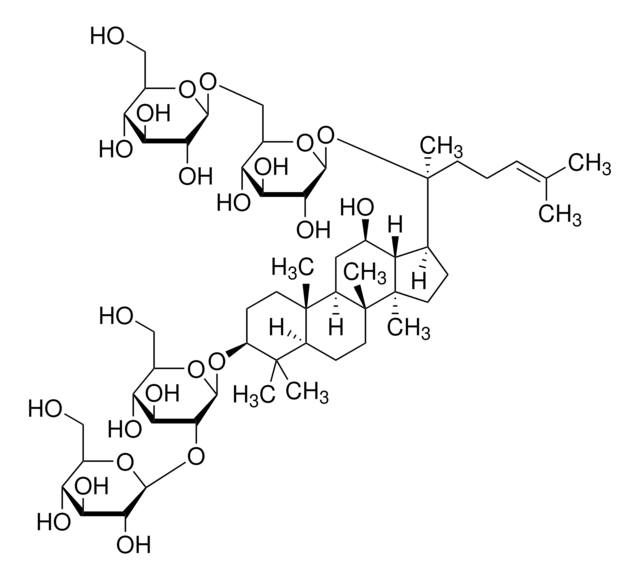
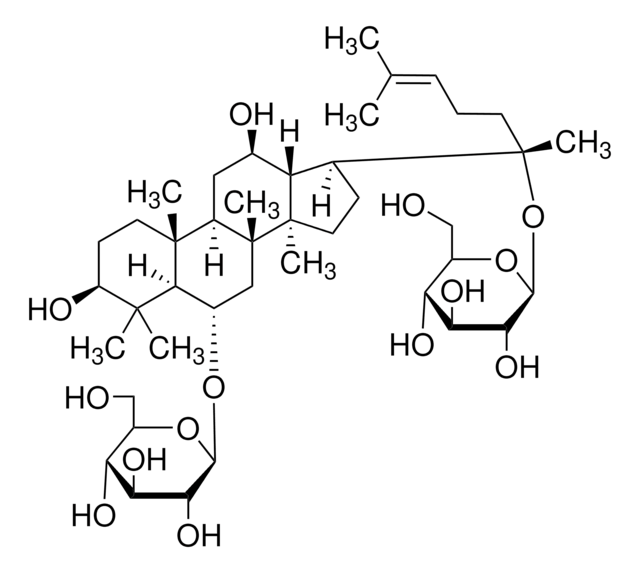
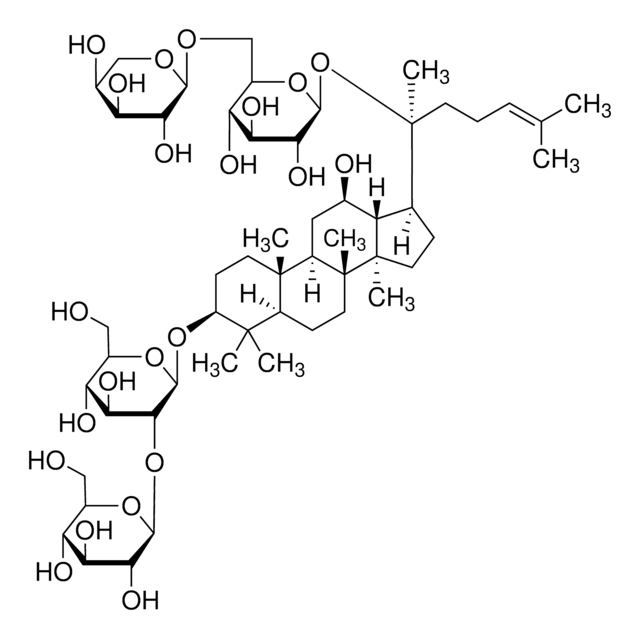
Ginsenoside Rf
primary reference standard
Sinônimo(s):
(3β,6α,12β)-3,12,20-Trihydroxydammar-24-en-6-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside, Panaxoside Rf
About This Item
Produtos recomendados
grau
primary reference standard
prazo de validade
limited shelf life, expiry date on the label
fabricante/nome comercial
HWI
aplicação(ões)
food and beverages
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
[H][C@]1(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@@]2([H])O[C@H]3C[C@]4(C)[C@]([H])(C[C@@H](O)[C@]5([H])[C@]([H])(CC[C@@]45C)[C@@](C)(O)CC\C=C(/C)C)[C@@]6(C)CC[C@H](O)C(C)(C)[C@]36[H]
InChI
1S/C42H72O14/c1-20(2)10-9-13-42(8,52)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-37-34(32(50)30(48)25(19-44)55-37)56-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24+,25+,26+,27-,28-,29+,30+,31-,32-,33+,34+,35-,36-,37+,39+,40+,41+,42-/m0/s1
chave InChI
UZIOUZHBUYLDHW-XUBRWZAZSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Exact content by quantitative NMR can be found on the certificate.
Aplicação
Outras notas
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
In this article we present several HPTLC applications and analytical standards for ginsenosides.
Conteúdo relacionado
Ginsenosides Separation in Ginseng. The HPLC method was first optimized using a ginsenoside standard mixture, and was then applied to a sample of American Ginseng root.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica