PF033
Fas Ligand, Human, Recombinant
Fas Ligand, Human, Recombinant, consists of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, rat, and mouse Fas.
Sinônimo(s):
CD95L, CD178
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
12352202
NACRES:
NA.77
Produtos recomendados
recombinante
expressed in HEK 293 cells
Nível de qualidade
Ensaio
≥95% (SDS-PAGE)
forma
lyophilized
potência
50 ng/mL ED50 (A20 cells)
não contém
preservative
reatividade de espécies
human, mouse, rat
fabricante/nome comercial
Calbiochem®
condição de armazenamento
OK to freeze
Impurezas
<0.1 EU/μg Endotoxin (EU/μg purified protein)
Condições de expedição
wet ice
temperatura de armazenamento
−20°C
Descrição geral
Recombinant, human, Fas ligand consisting of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, mouse, and rat Fas. The recombinant protein is produced in HEK293 cells. Useful for cytotoxicity assays.
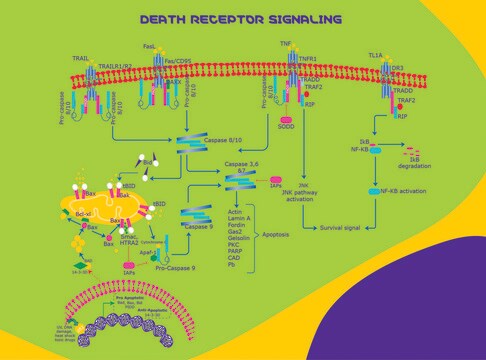
Fas/APO1/CD95 (37-42 kDa), a member of the Tumor Necrosis Factor/Nerve Growth Factor (TNF/NGF) receptor family, is an apoptosis-signaling surface receptor known to trigger apoptotic cell death. Homology exists between the Fas and TNF receptors including a region called the "death domain" which is required to propagate the apoptotic signal. The signal activates a family of cysteine proteases, or caspases, which systematically lead to cell destruction. Genetic defects in the Fas system have been shown to lead to autoimmune disorders while increased activity or deregulation contributes to the pathology of diseases such as AIDS.
Fas/APO1/CD95 (37-42 kDa), a member of the Tumor Necrosis Factor/Nerve Growth Factor (TNF/NGF) receptor family, is an apoptosis-signaling surface receptor known to trigger apoptotic cell death. Homology exists between the Fas and TNF receptors including a region called the "death domain" which is required to propagate the apoptotic signal. The signal activates a family of cysteine proteases, or caspases, which systematically lead to cell destruction. Genetic defects in the Fas system have been shown to lead to autoimmune disorders while increased activity or deregulation contributes to the pathology of diseases such as AIDS.
Recombinant, human, Fas ligand consisting of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, rat, and mouse Fas.The recombinant human soluble Fas ligand recognizes the Fas receptor in human, rat and mouse. Extracellular domain of human FasL, 103 - 281 fused at the N-terminus to a tag linker peptide.
Embalagem
Please refer to vial label for lot-specific concentration.
Advertência
Toxicity: Standard Handling (A)
forma física
Lyophilized from PBS.
Reconstituição
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Reconstitute in 50 μl H₂O to yield a final stock concentration of 100 µg/ml. Further dilute in culture medium containing 5% FCS immediately prior to use.
Outras notas
Noguchi, K. et al. 1996. Oncogene13, 39.
Golstein, P. et al. 1995. Cell81, 185.
Katsikis, P. et al. 1995. J. Exp. Med.181, 2029.
Nagata, S. et al. 1995. Science267, 1449.
Thompson, C., 1995. Science267, 1456.
Watanabe-Fukunaga, R. et al. 1992. Nature356, 314.
Golstein, P. et al. 1995. Cell81, 185.
Katsikis, P. et al. 1995. J. Exp. Med.181, 2029.
Nagata, S. et al. 1995. Science267, 1449.
Thompson, C., 1995. Science267, 1456.
Watanabe-Fukunaga, R. et al. 1992. Nature356, 314.
The soluble Fas ligand kills APO-1/Fas-sensitive cells at a concentration of >50 ng/ml. For example, cell death results when 5 x 105 murine A20 B lymphoma cells are incubated with 50-500 ng/ml of the recombinant human soluble APO1/FasL (aa 103-281) for 16 h at 37°C.
Informações legais
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica