401003
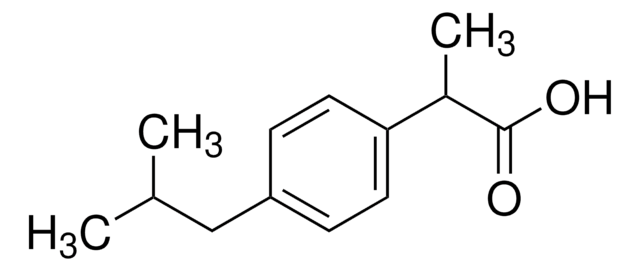
(±)-Ibuprofen
A nonsteroidal anti-inflammatory drug (NSAID) that acts as a reversible and competitive inhibitor of cyclooxygenase 1 (COX-1) (IC₅₀ = 4.85 µM).
Sinônimo(s):
(±)-Ibuprofen, [(±)-2-(4-Isobutylphenyl)-propionic Acid
About This Item
Produtos recomendados
Nível de qualidade
descrição
Merck USA index - 14, 4881
Ensaio
≥98% (titration)
Formulário
solid
fabricante/nome comercial
Calbiochem®
condição de armazenamento
OK to freeze
protect from light
cor
white
solubilidade
ethanol: 1 mg/mL
DMSO: 5 mg/mL
Condições de expedição
ambient
temperatura de armazenamento
10-30°C
cadeia de caracteres SMILES
OC(=O)C(C)c1ccc(cc1)CC(C)C
InChI
1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
chave InChI
HEFNNWSXXWATRW-UHFFFAOYSA-N
Descrição geral
Ações bioquímicas/fisiológicas
COX-1
Advertência
Reconstituição
Outras notas
Blasko, I., et al. 2001. Neurobiol. Dis.8, 1094.
Ouellet, M., et al. 2001. Proc. Natl. Acad. Sci. USA98, 14583.
Casper, D., et al. 2000. Neurosci. Lett.289, 201.
Lambat, Z., et al. 2000. Metab. Brain Dis.15, 249.
Lim, G.P., et al. 2000. J. Neurosci.20, 5709.
Ogawa, O., et al. 2000. Eur. J. Pharmacol.408, 137.
Wyss-Coray, T., and Mucke, L. 2000. Nat. Med.6, 973.
Lehmann, J.M., et al. 1997. J. Biol. Chem.272, 3406.
Boneburg, E.M., et al. 1996. J. Clin. Pharmacol.36, 16S.
Mitchell, J.A., et al. 1994. Proc. Natl. Acad. Sci. USA90, 11693.
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica