Z-004
Zaleplon solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produtos recomendados
grau
certified reference material
Formulário
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
embalagem
ampule of 1 mL
fabricante/nome comercial
Cerilliant®
concentração
1.0 mg/mL in methanol
técnica(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicação(ões)
clinical testing
Formato
single component solution
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
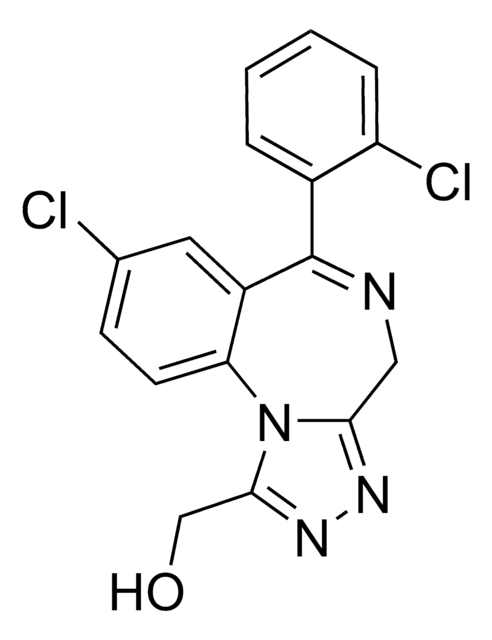
CCN(C(C)=O)c1cccc(c1)-c2ccnc3c(cnn23)C#N
InChI
1S/C17H15N5O/c1-3-21(12(2)23)15-6-4-5-13(9-15)16-7-8-19-17-14(10-18)11-20-22(16)17/h4-9,11H,3H2,1-2H3
chave InChI
HUNXMJYCHXQEGX-UHFFFAOYSA-N
Informações sobre genes
human ... GABRA1(2554) , GABRB1(2560) , GABRG2(2566)
Categorias relacionadas
Descrição geral
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Órgãos-alvo
Eyes
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
49.5 °F - closed cup
Ponto de fulgor (°C)
9.7 °C - closed cup
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
An rapid method for the siµLtaneous determination of the Z-drugs or sleep aids: zopiclone, zolpidem, and zaleplon is presented here. The need for greater analytical capacity and throughput for the analysis of sleep aid medicines (Z-drugs) in forensic toxicology laboratories can be met by the use of fast Ascentis Express 2.0 micron Fused Core UHPLC Columns.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica