850400C
Avanti
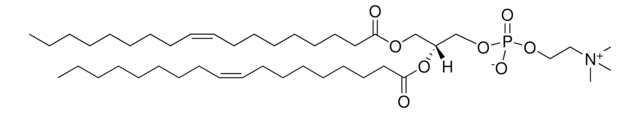
22:6 (Cis) PC
1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, chloroform
Sinônimo(s):
1,2-di-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphocholine; PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z))
About This Item
Produtos recomendados
Ensaio
>99% (TLC)
Formulário
liquid
embalagem
pkg of 1 × 2.5 mL (850400C-25mg)
pkg of 5 × 4 mL (850400C-500mg)
fabricante/nome comercial
Avanti Research™ - A Croda Brand 850400C
concentração
10 mg/mL (850400C-25mg)
25 mg/mL (850400C-500mg)
tipo de lipídio
cardiolipins
phospholipids
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
[P](=O)([O-])(OC[C@H](OC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)COC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)OCC[N+](C)(C)C
InChI
1S/C52H80NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-38-40-42-44-51(54)58-48-50(49-60-62(56,57)59-47-46-53(3,4)5)61-52(55)45-43-41-39-37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h8-11,14-17,20-23,26-29,32-35,38-41,50H,6-7,12-13,18-19,24-25,30-31,36-37,42-49H2,1-5H3/b10-8-,11-9-,16-14-,17-15-,22-20-,23-21-,28-26-,29-27-,34-32-,35-33-,40-38-,41-39-/t50-/m1/s1
chave InChI
XLKQWAMTMYIQMG-SVUPRYTISA-N
Descrição geral
Aplicação
- in liposomes, to study its effect on membrane vesiculation by dynamin and endophilin
- in multi-lamellar vesicles (MLVs) to analyze its effect on the biophysical properties of lipid membranes and on its interaction with a fragment of the Aβ peptide
- in lipid bilayers to study the influence of cholesterol on lateral segregation of saturated and unsaturated phospholipids
Embalagem
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Órgãos-alvo
Central nervous system, Liver,Kidney
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
does not flash
Ponto de fulgor (°C)
does not flash
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica