638080
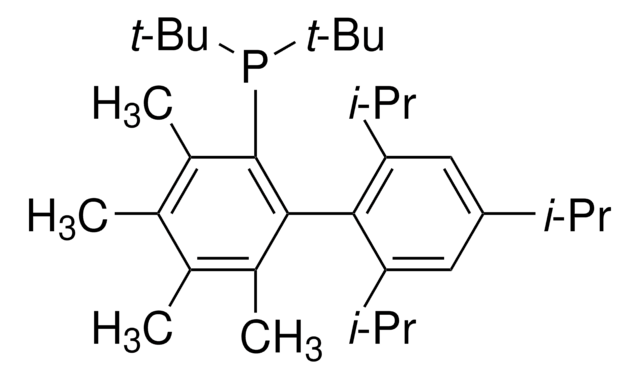
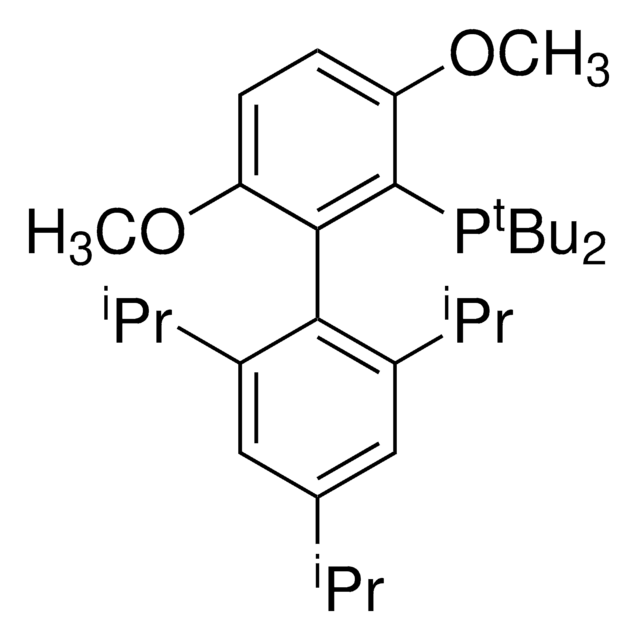
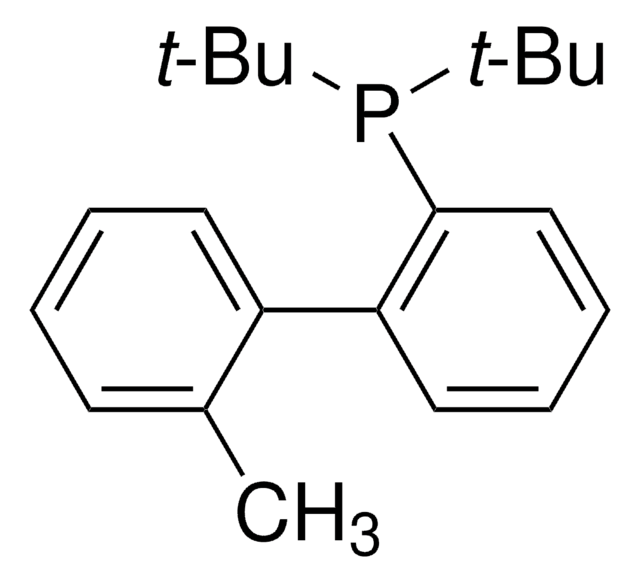
tBuXPhos
98%
Sinônimo(s):
tBuXPhos, 2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl, t-Bu XPhos, tert-Butyl XPhos
About This Item
Produtos recomendados
Ensaio
98%
Formulário
solid
adequação da reação
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carboxylations
reagent type: ligand
reaction type: Decarboxylations
pontuação do produto alternativo mais ecológico
old score: 12
new score: 1
Find out more about DOZN™ Scoring
características do produto alternativo mais ecológico
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
pf
148-151 °C (lit.)
grupo funcional
phosphine
categoria alternativa mais ecológica
cadeia de caracteres SMILES
CC(P(C(C=CC=C1)=C1C(C(C(C)C)=CC(C(C)C)=C2)=C2C(C)C)C(C)(C)C)(C)C
InChI
1S/C29H45P/c1-19(2)22-17-24(20(3)4)27(25(18-22)21(5)6)23-15-13-14-16-26(23)30(28(7,8)9)29(10,11)12/h13-21H,1-12H3
chave InChI
SACNIGZYDTUHKB-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Learn more about Buchwald Phosphine Ligands
Aplicação
It can be used in the following reactions:
- Palladium-catalyzed Tsuji-Trost substitution and cross-coupling of benzylic fluorides.
- Palladium-catalyzed C-N cross-coupling of sulfinamides and aryl halides.
- Palladium-catalyzed rapid methoxylation and deuteriomethoxylation of bromo-chalcones.
Informações legais
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Buchwald Phosphine Ligands
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica