About This Item
Fórmula empírica (Notação de Hill):
C9H6ClNO
Número CAS:
Peso molecular:
179.60
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
Produtos recomendados
Ensaio
98%
pf
213-216 °C (lit.)
grupo funcional
aldehyde
chloro
cadeia de caracteres SMILES
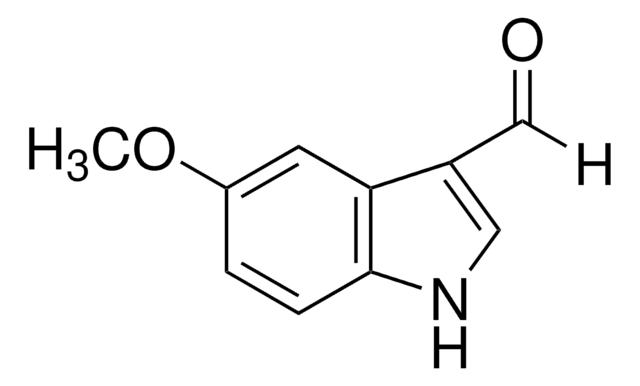
Clc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C9H6ClNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
chave InChI
YXEXOIGXNYITQH-UHFFFAOYSA-N
Descrição geral
5-Chloroindole-3-carboxaldehyde, also known as 5-chloro-1H-indole-3-carboxaldehyde, is an indole derivative.
Aplicação
5-Chloroindole-3-carboxaldehyde (5-Chloro-1H-indole-3-carboxaldehyde) may be used in the preparation of:
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde isonicotinoyl hydrazine
- 2′-[(5-chloro-1H-indol-3-yl)methylene]-2-(1H-indol-3-yl)acetohydrazide
- 5-chloro-3-(2,2-dibromovinyl)-1-(2-trimethylsilylethoxymethyl)indole
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde 3-chlorobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-nitrobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 3-methylbenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-methylbenzoylhydrazone
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Electrochemical behavior of indole-3-carboxaldehyde izonicotinoyl hydrazones: discussion on possible biological behavior
Shirinzadeh H, et al.
Combinatorial Chemistry & High Throughput Screening, 13(7), 619-627 (2010)
Tandem Suzuki-Miyaura cross-coupling/dehydrobromination of 1, 1-dibromoalkenes to alkynes with a cyclobutene-1, 2-diylbis (imidazolium) salt as catalyst precursor.
Rahimi A and Schmidt A.
Synthesis, 2010(15), 2621-2625 (2010)
2?-[(5-Chloro-1H-indol-3-yl) methylene]-2-(1H-indol-3-yl) acetohydrazide.
Ali HM, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(4), o1807-o1808 (2007)
Kamaleddin Haj Mohammad Ebrahim Tehrani et al.
Iranian journal of pharmaceutical research : IJPR, 14(4), 1077-1086 (2015-12-15)
A series of indole-based aryl(aroyl)hydrazone analogs of antiplatelet indole-3-carboxaldehyde phenylhydrazone were synthesized by the Schiff base formation reaction and their antiplatelet activity was assessed using human platelet rich plasma. The platelet concentrate was obtained using a two-step centrifugation protocol and
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica