262439
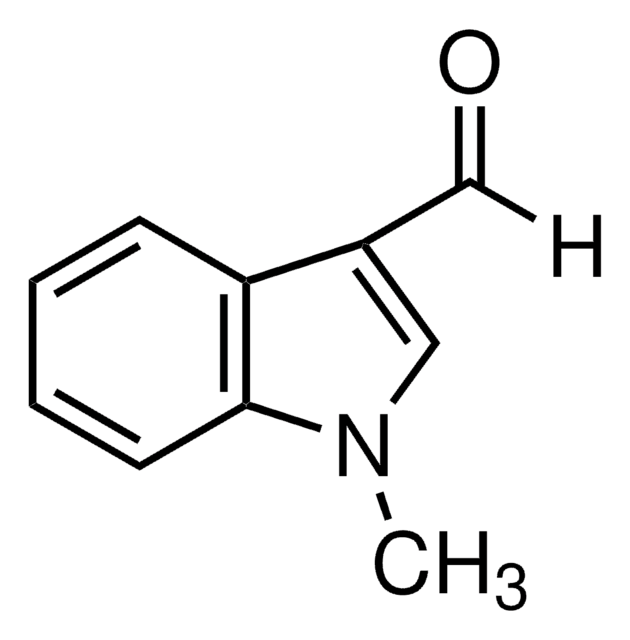
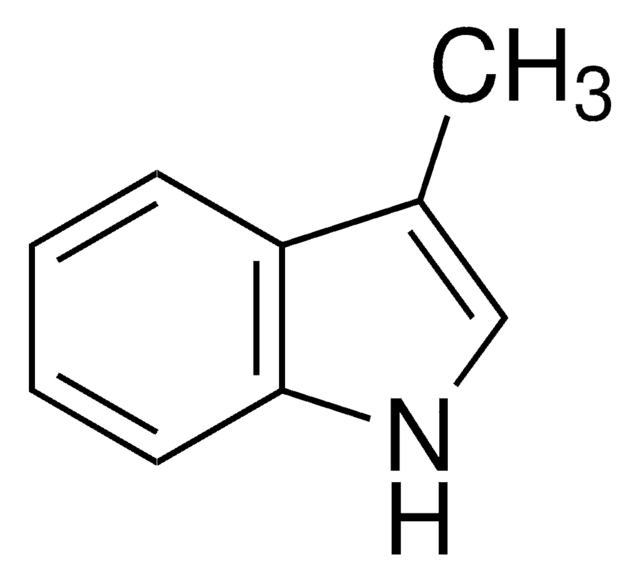
2-Methylindole-3-carboxaldehyde
97%
Sinônimo(s):
3-Formyl-2-methylindole, NSC 11895
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H9NO
Número CAS:
Peso molecular:
159.18
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
200-201 °C (lit.)
grupo funcional
aldehyde
cadeia de caracteres SMILES
Cc1[nH]c2ccccc2c1C=O
InChI
1S/C10H9NO/c1-7-9(6-12)8-4-2-3-5-10(8)11-7/h2-6,11H,1H3
chave InChI
CYZIVXOEJNAIBS-UHFFFAOYSA-N
Descrição geral
Oxidative activation of 2-methylindole-3-carboxaldehyde via N-heterocyclic carbene organocatalysis generates heterocyclic ortho-quinodimethane as a key intermediate.
Aplicação
2-Methylindole-3-carboxaldehyde has been used in the preparation of 1-phenylsulfonyl-2-methylindole-3-carboxaldehyde.
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Fluorescent sensors (BODIPY)
- Antimicrobial agents against methicillin-resistant Staphylococcus aureus
- G protein-coupled receptor CRTh2 antagonists
- Inhibitors of PI3 kinase-α
- Antitubercular agents
- Anti-inflammatory agents
- Mycobacterium tuberculosis protein tyrosine phosphatase B
- Glucocorticoid receptor ligands
- Agents stimulating neurite outgrowth
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Xingkuan Chen et al.
Angewandte Chemie (International ed. in English), 52(42), 11134-11137 (2013-09-17)
Aryl aldehyde activation: Oxidative activation of 2-methylindole-3-carboxaldehyde (I) through N-heterocyclic carbene (NHC) organocatalysis generates heterocyclic ortho-quinodimethane (II) as a key intermediate. This intermediate then undergoes formal [4+2] cycloaddition with trifluoromethyl ketones or isatins to form polycyclic lactones containing a quaternary
G Chakkaravarthi et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o542-o542 (2008-01-01)
In the title compound, C(16)H(15)NO(3)S, the plane of the phenyl ring forms a dihedral angle of 80.37 (8)° with the indole ring system. The crystal packing is stabilized by weak O-H⋯O hydrogen bonds which link the mol-ecules into infinite chains along
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica