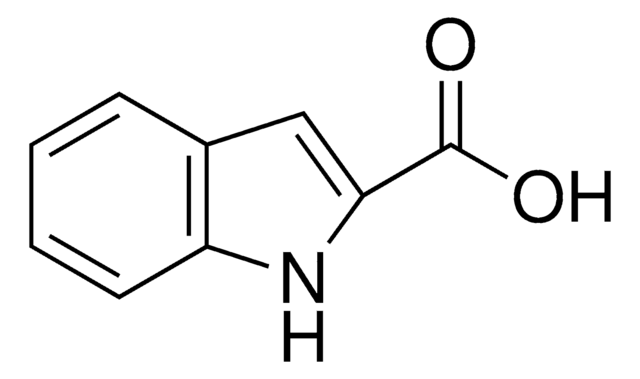
511129
1-Methylindole-2-carboxaldehyde
97%
Sinônimo(s):
2-Formyl-1-methylindole, NSC 106285
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H9NO
Número CAS:
Peso molecular:
159.18
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
81-85 °C (lit.)
grupo funcional
aldehyde
cadeia de caracteres SMILES
Cn1c(C=O)cc2ccccc12
InChI
1S/C10H9NO/c1-11-9(7-12)6-8-4-2-3-5-10(8)11/h2-7H,1H3
chave InChI
IBNGPIOSWCMJGG-UHFFFAOYSA-N
Aplicação
1-Methylindole-2-carboxaldehyde may be used in the synthesis of indole-based melatonin analog hydrazone derivatives and (E)-5-((benzyloxy)methyl)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-3-((1-methyl-1H-indol-2-yl)methylene)dihydrofuran-2(3H)-one.
Reactant for preparation of:
- Deazapurine isosteres from acylindoles via pyridine ring annulation
- 2,4-dichlorocinnamohydroxamic acid analogs for enhancing pharmacokinetics of botulinum neurotoxin serotype A protease inhibitors
- Tetrahydrocarbazoles via organocatalytic cascade Friedel-Crafts alkylation/Michael addition/aromatization reaction
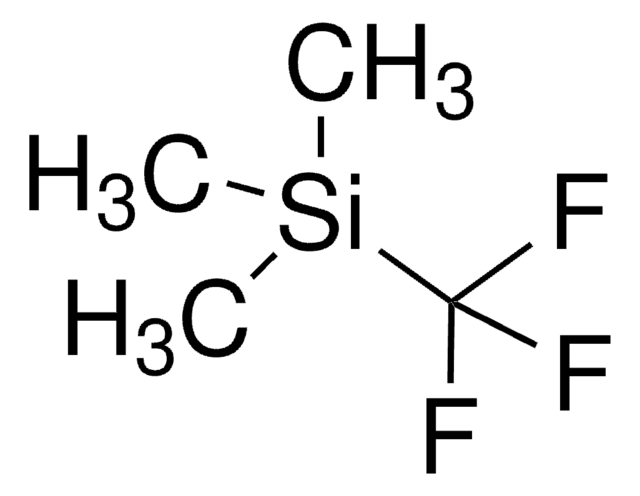
- Azides, imines and amines via flow processes of amines and trimethylsilyl azide and aza-Wittig reaction
- 3-indolylpyridinedicarbonitriles as anti-inflammatory agents
- Bis(indolyl)methanes as antimicrobial and antioxidant agents
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Sibel Suzen et al.
Journal of enzyme inhibition and medicinal chemistry, 28(6), 1143-1155 (2012-09-22)
Melatonin (MLT) is a strong free-radical scavenger, which protects the body from the effects of oxidants. In recent years, MLT have been described resulting in much attention in the development of synthetic compounds possessing. As a part of our ongoing
Noga Gal et al.
Chembiochem : a European journal of chemical biology, 12(15), 2331-2340 (2012-10-30)
N-methyl-substituted diacylglycerol-indololactones (DAG-indololactones) are newly synthesized effectors of protein kinase C (PKC) isoforms and exhibit substantial selectivity between RasGRP3 and PKCα. We present a comprehensive analysis of membrane interactions and biological activities of several DAG-indololactones. Translocation and binding activity assays
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica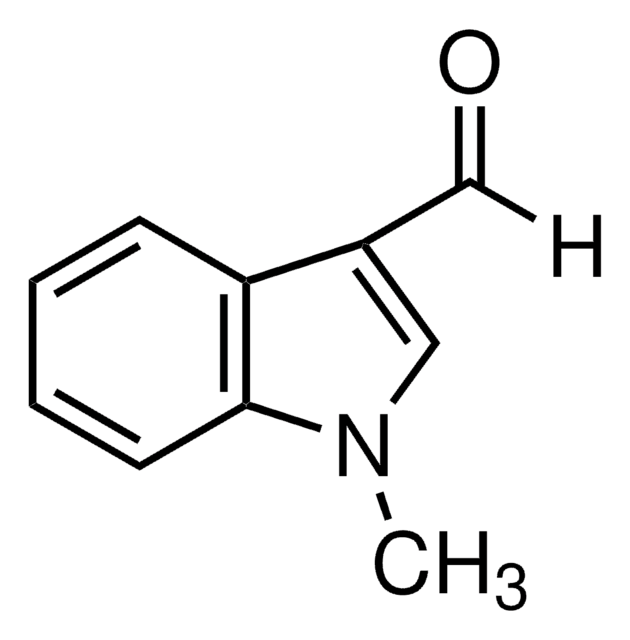

![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)