About This Item
Fórmula empírica (Notação de Hill):
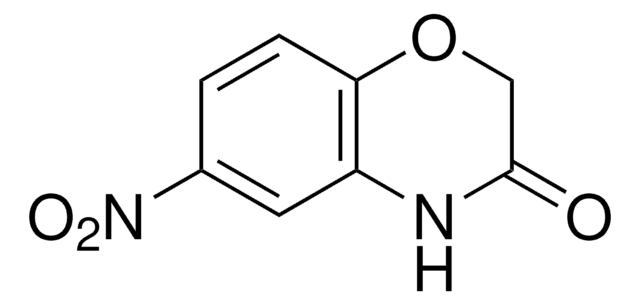
C8H7NO2
Número CAS:
Peso molecular:
149.15
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
pf
173-175 °C (lit.)
solubilidade
methanol: soluble 25 mg/mL, clear, colorless
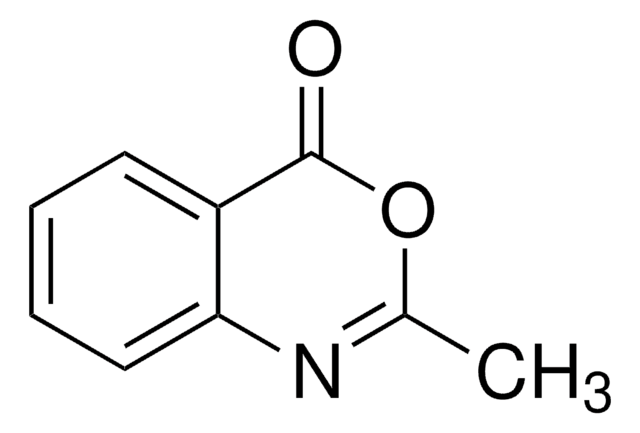
cadeia de caracteres SMILES
O=C1COc2ccccc2N1
InChI
1S/C8H7NO2/c10-8-5-11-7-4-2-1-3-6(7)9-8/h1-4H,5H2,(H,9,10)
chave InChI
QRCGFTXRXYMJOS-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
2H-1,4-Benzoxazin-3(4H)-one, a benzoxazine derivative, is a heterocyclic building block for various natural and synthetic organic compounds. It has been reported as an intermediate during the biogenesis of cyclic hydoxamic acids in maize. Its standard molar enthalpy of formation and tautomerization energy of its tautomers has been evaluated by calorimetric and computational methods. It has been synthesized by reacting o-aminophenol with chloroacetyl chloride in the presence of butanone and aqueous NaHCO3.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
2H-1,4-benzoxazin-3(4H)-one, an intermediate in the biosynthesis of cyclic hydroxamic acids in maize.
Kumar P, et al.
Phytochemistry, 36(4), 893-898 (1994)
A general and convenient synthesis of 2H-1,4-benzoxazin-3(4H)-ones.
Shridhar DR, et al.
Organic Prep. and Proc. Int., 14(3), 195-197 (1982)
Jiu Hong Wu et al.
Bioorganic & medicinal chemistry letters, 13(13), 2223-2225 (2003-06-12)
A new inhibitor of in vitro tumor cell replication, cappamensin A (1) (2H-1,4-benzoxazin-3(4H)-one, 6-methoxy-2-methyl-4-carbaldehyde), was isolated from the roots of Capparis sikkimensis subsp. formosana using bioactivity-guided fractionation. The structure of 1 was established by spectroscopic methods, including 2D NMR analyses.
Calorimetric and computational study of 2H-1,4-benzoxazin-3(4H)-one and of related species.
Matos MAR, et al.
Molecular Physics, 104(12), 1833-1841 (2006)
Recent advances in the synthesis of 2H-1, 4-benzoxazin-3-(4H)-ones and 3, 4-dihydro-2 H -1, 4-benzoxazines.
Ilas J, et al.
Tetrahedron, 61(31), 7325-7348 (2005)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica