210145
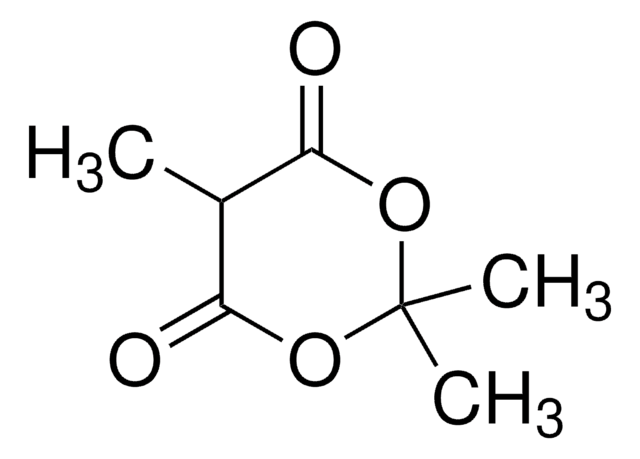
2,2-Dimethyl-1,3-dioxane-4,6-dione
98%
Sinônimo(s):
Malonic acid cyclic isopropylidene ester, Meldrum’s acid, cycl-Isopropylidene malonate
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
solid
pf
92-96 °C (lit.)
solubilidade
dioxane: soluble 5%, clear to very slightly hazy, colorless to faintly yellow
grupo funcional
ester
ketal
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CC1(C)OC(=O)CC(=O)O1
InChI
1S/C6H8O4/c1-6(2)9-4(7)3-5(8)10-6/h3H2,1-2H3
chave InChI
GXHFUVWIGNLZSC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Meldrum′s acid is used as a valuable starting material to synthesize heterocycles and as intermediates in organic synthesis reactions.
Aplicação
- macrocyclic β-keto lactone
- 4-pyridyl-substituted heterocycles
- 2-substituted indoles
- isofraxidin.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
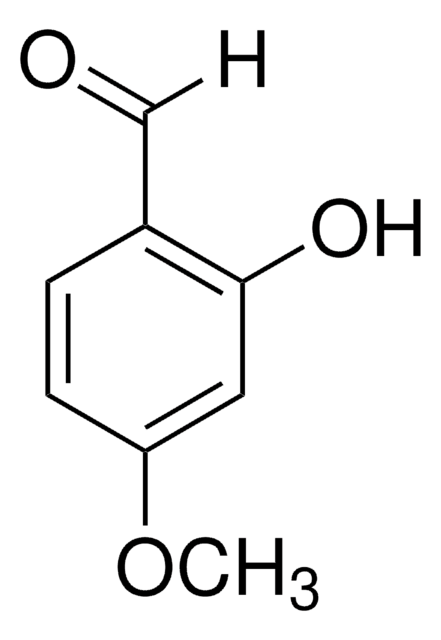
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica