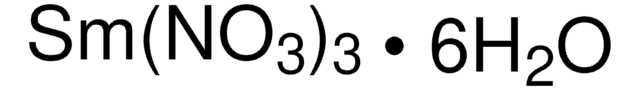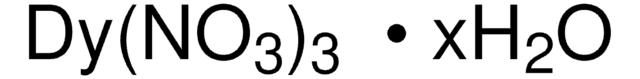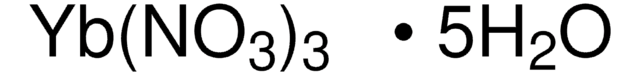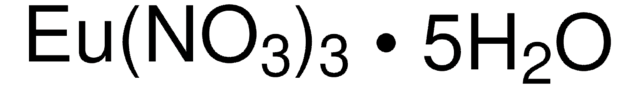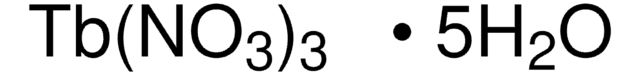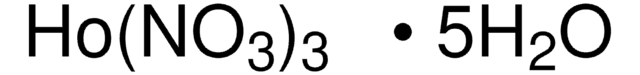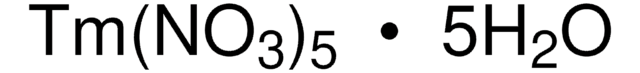205133
Praseodymium(III) nitrate hexahydrate
99.9% trace metals basis
Sinônimo(s):
Praseodymium trinitrate hexahydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
Ensaio
99.9% trace metals basis
Formulário
crystalline
adequação da reação
reagent type: catalyst
core: praseodymium
Impurezas
≤2000 ppm Trace Metal Analysis
cadeia de caracteres SMILES
O.O.O.O.O.O.[Pr+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/3NO3.6H2O.Pr/c3*2-1(3)4;;;;;;;/h;;;6*1H2;/q3*-1;;;;;;;+3
chave InChI
LXXCECZPOWZKLC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- A dopant to fabricate dye-sensitized solar cells. The addition of rare earth enhances the power conversion efficiency of solar cells by narrowing the band gap of photoanode materials.
- A precursor to synthesize high entropy lanthanide oxysulfides ( wide band gap semiconductors).
- To synthesize functionalized UV-emitting nanocomposite for photodynamic cancer therapy.
- To fabricate Pr-doped MoO3 thinfilms for gas sensing applications.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2
Código de classe de armazenamento
5.1B - Oxidizing hazardous materials
Classe de risco de água (WGK)
WGK 2
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 205133-250G | 4061838767479 |
| 205133-10G | 4061838767462 |
| 205133-50G | 4061838767486 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica