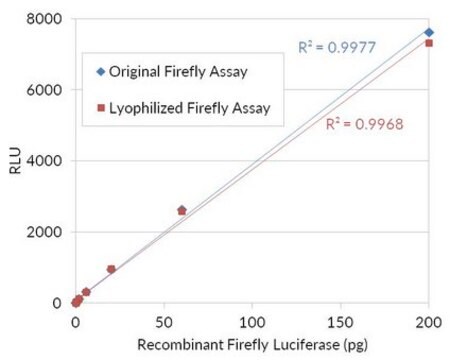
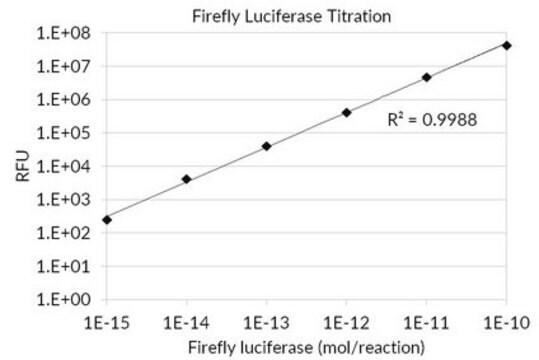
SRE0045
Luciferase from Photinus pyralis (firefly)
recombinant, expressed in E. coli, lyophilized powder, ≥10×1010 units/mg protein
Synonyme(s) :
Luciferase firefly
About This Item
Produits recommandés
Produit recombinant
expressed in E. coli
Niveau de qualité
Forme
lyophilized powder
Activité spécifique
≥10×1010 units/mg protein
Poids mol.
62 kDa
Application(s)
diagnostic assay manufacturing
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Actions biochimiques/physiologiques
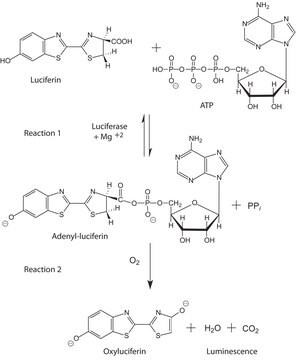
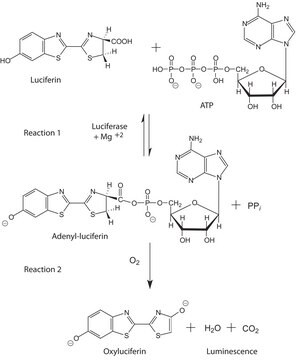
Définition de l'unité
Unit Definition Conversion Factor: There are approximately 9000 Relative Light Units (RLU) per one traditional Light Unit that uses a peak height equivalent to 0.02 μCi of 14C in a PPO/POPOP cocktail.
Forme physique
Notes préparatoires
After reconstitution, the enzyme solutions can kept at 4-8 °C for up to 2 days or frozen in working aliquots at -20°C for at least one month. Repeated freezing and thawing is not recommended.
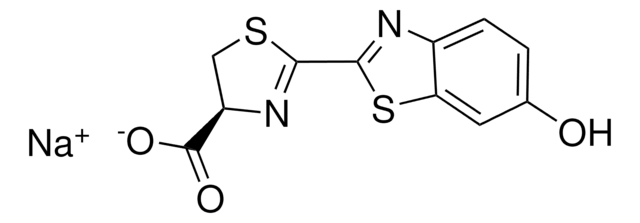
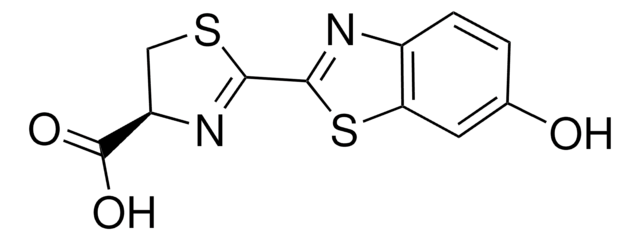
Substrat
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Firefly luciferase is a widely used bioluminescent reporter for studying gene regulation and function. It is a very sensitive genetic reporter due to the absence of endogenous luciferase activity in mammalian cells or tissues.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique