SML2591
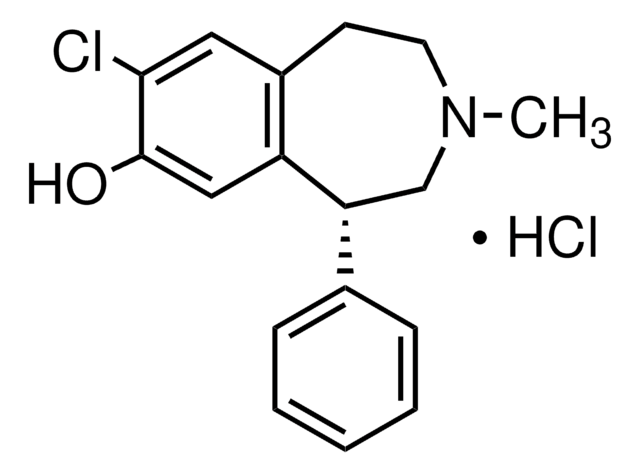
SCH-39166 hydrobromide
≥98% (HPLC)
Synonyme(s) :
(-)-trans-6,7,7a,8,9,13b-Hexahydro-3-chloro-2-hydroxy-N-methyl- 5H-benzo[d]naptho-(2,1-b)azepine hydrobromide, (6aS,13bR)-11-Chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepin-12-ol hydrobromide, (6aS-trans)-11-Chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-Benzo[d]naphth[2,1-b]azepin-12-ol hydrobromide, Ecopipam hydrobromide, PSYRX 101 hydrobromide, PSYRX-101 hydrobromide, PSYRX101 hydrobromide, SCH 39166 hydrobromide, SCH39166 hydrobromide
About This Item
Produits recommandés
Essai
≥98% (HPLC)
Forme
powder
Conditions de stockage
desiccated
Couleur
white to beige
Solubilité
DMSO: 2 mg/mL, clear
Température de stockage
2-8°C
Actions biochimiques/physiologiques
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique