SML0562
Shiga Toxin 1, B subunit
recombinant, expressed in E. coli, ≥95% (SDS-PAGE)
Synonyme(s) :
SLT1, STX1, STxB
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352200
Nomenclature NACRES :
NA.77
Produits recommandés
Produit recombinant
expressed in E. coli
Niveau de qualité
Pureté
≥95% (SDS-PAGE)
Forme
lyophilized
Conditions d'expédition
dry ice
Température de stockage
−20°C
Application
Shiga Toxin 1, B subunit has been used to induce globotriaosylceramide (Gb3) endocytosis.
Actions biochimiques/physiologiques
The Shiga toxins are a family of related protein toxins secreted by certain types of bacteria. Shiga toxin (Stx) is produced by Shigella dysenteriae, whereas the Shiga-like toxins, Stx1 and Stx2, with a few known isoforms, are secreted by specific strains of Escherichia coli named Shiga-toxin-producing E. coli (STEC) such as E. coli O157:H7, which causes bloody diarrhea and hemorrhagic colitis in humans, sometimes resulting in fatal systemic complications.
Stx1 is identical to Stx, while the Stx2 isoforms share less sequence similarity with Stx (~60%) and are immunologically distinct. In spite of the differences in their amino acid sequence, all Stx isoforms share the same overall toxin structure and mechanism of action.
Shiga toxins consist of two polypeptides: An A chain and a B chain non-covalently associated with an apparent stoichiometry of one A and five B chains, to form the holotoxin. The catalytic A subunit has a RNA N-glycosidase activity that inhibits eukaryotic protein synthesis. The B subunits form a pentamer that recognizes and binds to the functional cell-surface receptor globotriaosylceramide [Gb3; Gala(1-4)-Galb(1-4)-Glcb1-ceramide]. Gb3 is overexpressed in membranes of numerous tumor cells, therefore STxB binding to Gb3 receptors may be useful for cell-specific vectorization, labeling and imaging purposes.
Stx1 is identical to Stx, while the Stx2 isoforms share less sequence similarity with Stx (~60%) and are immunologically distinct. In spite of the differences in their amino acid sequence, all Stx isoforms share the same overall toxin structure and mechanism of action.
Shiga toxins consist of two polypeptides: An A chain and a B chain non-covalently associated with an apparent stoichiometry of one A and five B chains, to form the holotoxin. The catalytic A subunit has a RNA N-glycosidase activity that inhibits eukaryotic protein synthesis. The B subunits form a pentamer that recognizes and binds to the functional cell-surface receptor globotriaosylceramide [Gb3; Gala(1-4)-Galb(1-4)-Glcb1-ceramide]. Gb3 is overexpressed in membranes of numerous tumor cells, therefore STxB binding to Gb3 receptors may be useful for cell-specific vectorization, labeling and imaging purposes.
Forme physique
The recombinant product is Shiga toxin 1, B subunit, a 7 kDa protein containing 69 amino acid residues. It is lyophilized from 0.2 μm-filtered solution of phosphate buffer without any carrier protein.
Notes préparatoires
Reconstitute the contents of the vial using water to a concentration of 0.1-1.0 mg/mL. This solution can then be further diluted into other aqueous buffers and stored at 2-8 °C for up to 4 months or at −20 °C for extended use.
Remarque sur l'analyse
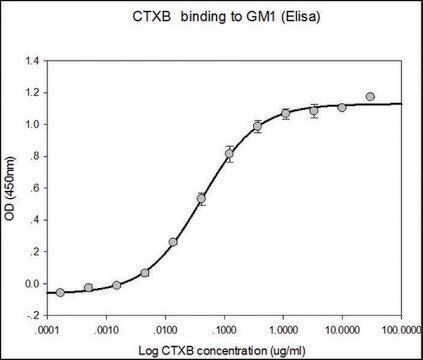
Gb3 Binding activity: significant binding above background is achieved with ≤1 μg/mL of STxB. The activity of STxB is measured by its ability to bind to Gb3, which requires its pentameric form.
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
The Pseudomonas aeruginosa lectin LecA triggers host cell signalling by glycosphingolipid-dependent phosphorylation of the adaptor protein CrkII
Zheng S, et al.
Biochimica et Biophysica Acta, 1864(7), 1236-1245 (2017)
In-Sun Shin et al.
Chemical & pharmaceutical bulletin, 54(4), 522-527 (2006-04-06)
A two-step binding assay for globotriaosylceramide (Gb3) content was developed by histidine-tagging strategy, which is a well-established method for the purification of recombinant proteins. The complete binding of the recombinant His-tagged Shiga toxin 1B subunit (1B-His) (1 microg/ml) to the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique