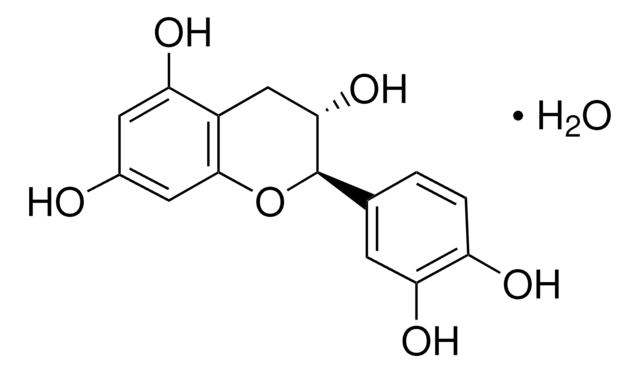
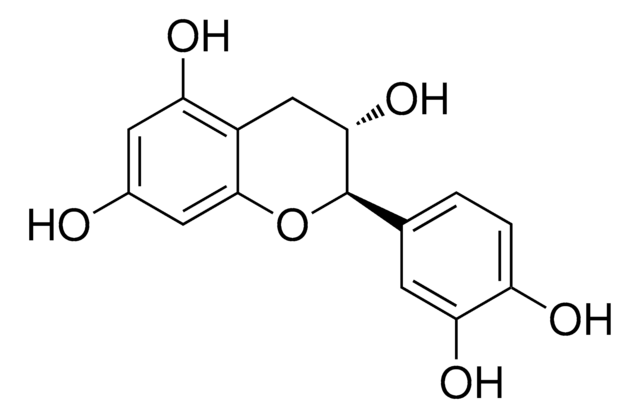
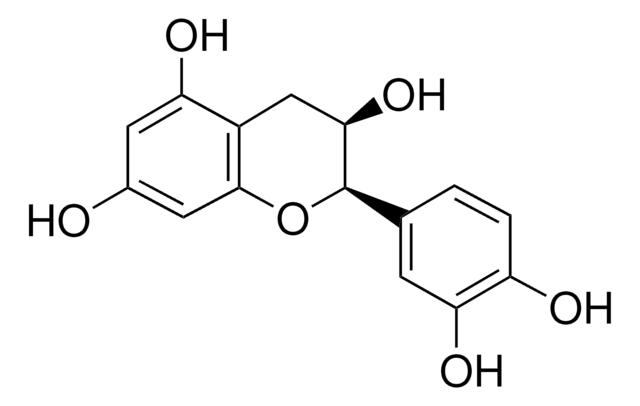
C1251
(+)-Catechin hydrate
≥98% (HPLC), powder
Synonyme(s) :
(+)-Cyanidol-3
About This Item
Produits recommandés
Pureté
≥98% (HPLC)
Forme
powder
Couleur
yellow to yellow with tan cast
Pf
175-177 °C (anhydrous) (lit.)
Solubilité
ethanol: 50 mg/mL
Température de stockage
2-8°C
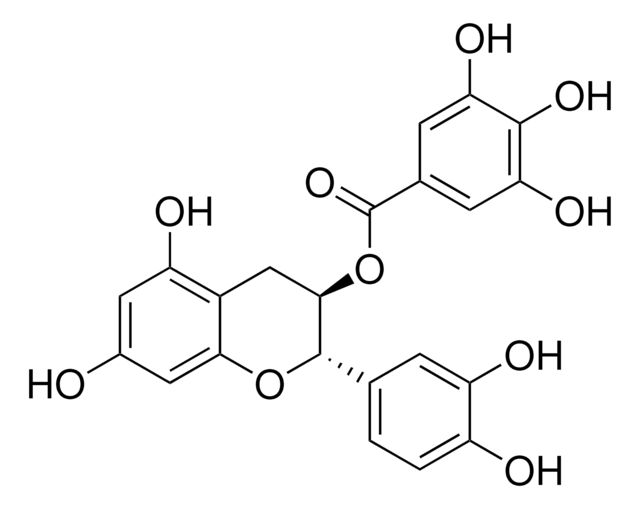
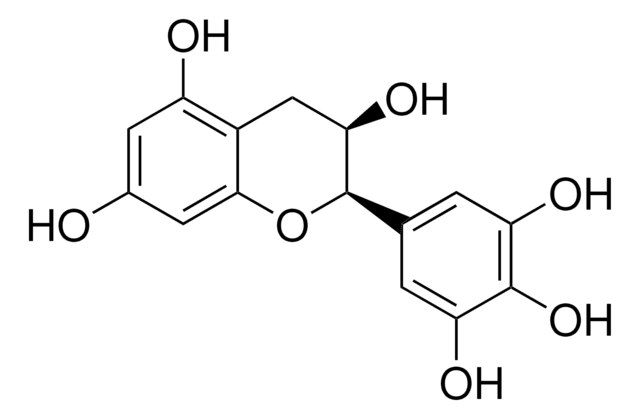
Chaîne SMILES
[H]O[H].O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3
InChI
1S/C15H14O6.H2O/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-5,13,15-20H,6H2;1H2/t13-,15+;/m0./s1
Clé InChI
OFUMQWOJBVNKLR-NQQJLSKUSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- as a polyphenol standard in the determination of total polyphenols in the by-products of red wine
- as an additive to study its effects on in vitro methane production and substrate degradation in a triple-fed batch approach
- as a substrate to determine the activity of pure L. plantarum CECT 748T 14 recombinant tannase on catechin
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocoles
Coumaric acid; Quercitrin; Myricetin; Quercetin
Contenu apparenté
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique