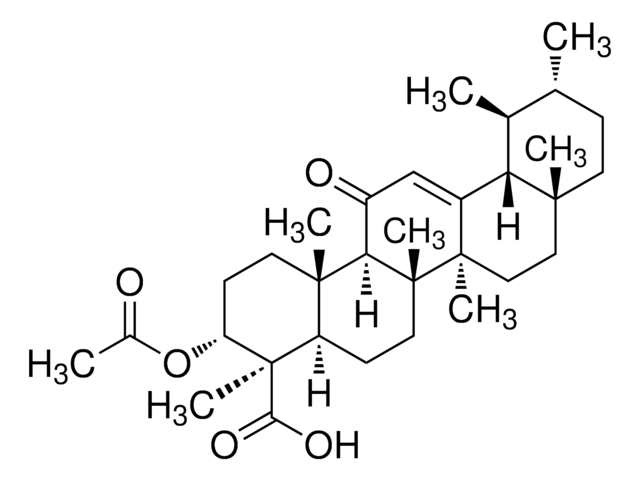
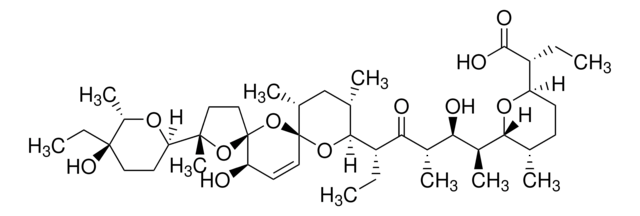
A9855
3-Acetyl-11-keto-BETA-boswellic acid
from Boswellia serrata
Synonyme(s) :
AKBA
About This Item
Produits recommandés
Source biologique
Boswellia serrata
Niveau de qualité
Forme
powder
Application(s)
metabolomics
vitamins, nutraceuticals, and natural products
Température de stockage
−20°C
Chaîne SMILES
O([C@H]1[C@]([C@H]2[C@@]([C@@H]3[C@]([C@@]4(CC[C@@]5([C@@H]([C@H]([C@@H](CC5)C)C)C4=CC3=O)C)C)(CC2)C)(CC1)C)(C)C(=O)O)C(=O)C
InChI
1S/C32H48O5/c1-18-9-12-28(4)15-16-30(6)21(25(28)19(18)2)17-22(34)26-29(5)13-11-24(37-20(3)33)32(8,27(35)36)23(29)10-14-31(26,30)7/h17-19,23-26H,9-16H2,1-8H3,(H,35,36)/t18-,19+,23-,24-,25+,26-,28-,29+,30-,31-,32-/m1/s1
Clé InChI
HMMGKOVEOFBCAU-BCDBGHSCSA-N
Description générale
Application
- to test its effects as an anti-proliferating agent on hepatic stellate cells (HSCs) proliferation and synergism in combination with imatinib in in vitro models
- to study its anti-osteoporotic activity on ovariectomy-induced osteoporosis in female Sprague Dawley rats
- in a comparative study with aspirin to test its effects as an anti-inflammatory drug to prevent intestinal adenomatous polyposis in APCMin/+ mice
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique