45999
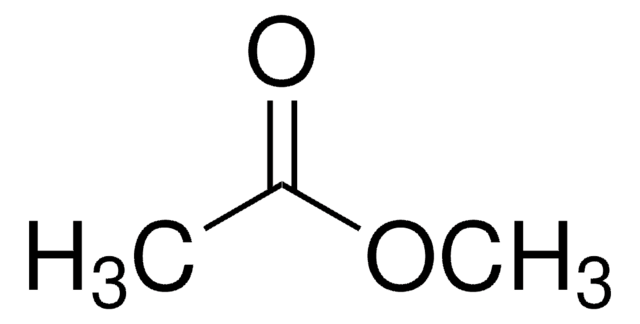
Methyl acetate
suitable for HPLC, ≥99.8%
About This Item
Produits recommandés
Densité de vapeur
2.55 (vs air)
Niveau de qualité
Pression de vapeur
165 mmHg ( 20 °C)
Pureté
≥99.8% (GC)
≥99.8%
Forme
liquid
Température d'inflammation spontanée
936 °F
Durée de conservation
limited shelf life, expiry date on the label
Limite d'explosivité
16 %
Technique(s)
HPLC: suitable
Impuretés
≤0.05% water
Résidus d'évap.
≤0.001%
Indice de réfraction
n20/D 1.361 (lit.)
n20/D 1.362
Point d'ébullition
57-58 °C (lit.)
Pf
−98 °C (lit.)
Densité
0.934 g/mL at 25 °C
Force du solvant ε (Al2O3)
0.60
λ
1 cm path, H2O reference
Absorption UV
λ: 255 nm Amax: 1.0
λ: 275 nm Amax: 0.1
λ: 300 nm Amax: 0.01
Chaîne SMILES
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
Clé InChI
KXKVLQRXCPHEJC-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Agglomeration of celecoxib by quasi-emulsion solvent diffusion method without stabilizer: effect of good solvent.: This research explored the agglomeration of celecoxib using the quasi-emulsion solvent diffusion method, highlighting the role of methyl acetate as a good solvent to improve particle size and distribution without the need for stabilizers (Maghsoodi & Nokhodchi, 2018).
- Formulation and in vitro evaluation of ketoprofen in palm oil esters nanoemulsion for topical delivery.: The formulation and evaluation of ketoprofen in a nanoemulsion system for topical delivery were examined, with methyl acetate used as a solvent to enhance solubility and stability of the active ingredient (Sakeena et al., 2010).
- Preparation of surfactant-free nanoparticles of methacrylic acid copolymers used for film coating.: This paper presented a method for preparing surfactant-free nanoparticles using methyl acetate as a solvent, emphasizing its role in the formation of uniform nanoparticle dispersions for coating applications (Nguyen et al., 2006).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Organes cibles
Central nervous system
Risques supp
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
8.6 °F - closed cup
Point d'éclair (°C)
-13 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Butyl methyl ether; Acetic acid; 2-Butanone; Ethyl acetate; Tetrahydrofuran; 1-Butanol; Isopropyl acetate; Heptane; Propyl acetate; 3-Methylbutanol; 4-Methyl-2-pentanone; Isobutyl acetate; Butyl acetate; Dimethyl sulfoxide; Anisole; Cumene
Protocoles
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate
GC Analysis of Class 3 Residual Solvents on SUPELCOWAX® 10
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique