W318101
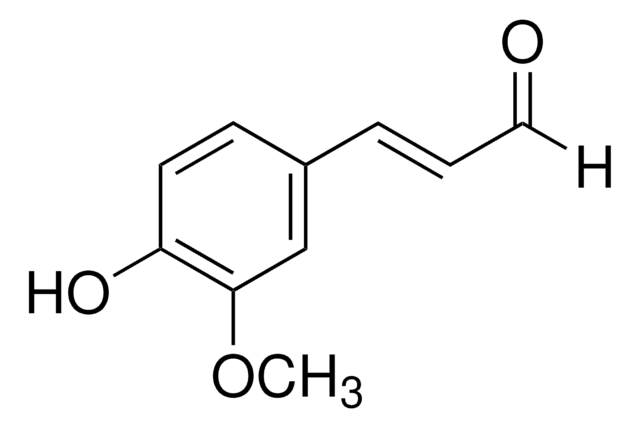
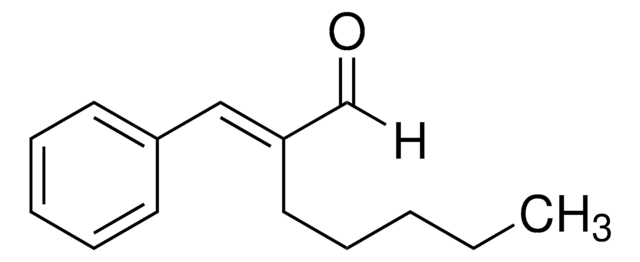
2-Methoxycinnamaldehyde
natural, 98%, FG
Synonyme(s) :
o-Methoxycinnamaldehyde
About This Item
Produits recommandés
Qualité
FG
Fragrance grade
Halal
Kosher
natural
Niveau de qualité
Agence
follows IFRA guidelines
Conformité réglementaire
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Essai
98%
Caractéristiques du produit alternatif plus écologique
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
pb
160-161 °C/12 mmHg (lit.)
Pf
44.0-49.0 °C (lit.)
Application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
Allergène alimentaire
no known allergens
Allergène de parfum
no known allergens
Autre catégorie plus écologique
Propriétés organoleptiques
cinnamon; woody; spicy; sweet
Chaîne SMILES
[H]C(=O)C=Cc1ccccc1OC
InChI
1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+
Clé InChI
KKVZAVRSVHUSPL-GQCTYLIASA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.: This study investigates the anti-inflammatory potential of 2-Methoxycinnamaldehyde in Cinnamomi ramulus. Its application extends to novel therapeutic approaches for treating ankylosing spondylitis, demonstrating significant implications for medicinal chemistry and pharmacology (Wei et al., 2023).
- Ramulus Cinnamomi essential oil exerts an anti-inflammatory effect on RAW264.7 cells through N-acylethanolamine acid amidase inhibition.: The study elaborates on the anti-inflammatory activities of 2-Methoxycinnamaldehyde, offering a biochemical pathway that could be exploited in anti-inflammatory drug design (Jia et al., 2023).
- Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.: This article offers insights into the antibacterial properties of 2-Methoxycinnamaldehyde, using metabolomics to explore its mechanism of action, significant for developments in antimicrobial treatments (Qian et al., 2022).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
235.4 °F
Point d'éclair (°C)
113 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Global Trade Item Number
| Référence | GTIN |
|---|---|
| W318101-100G-K | 4061837528002 |
| W318101-1KG-K | 4061834365853 |
| W318101-10KG-K | 4061833905906 |
| W318101-25KG-K | 4061833905913 |
| W318101-SAMPLE-K | 4061834356080 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique