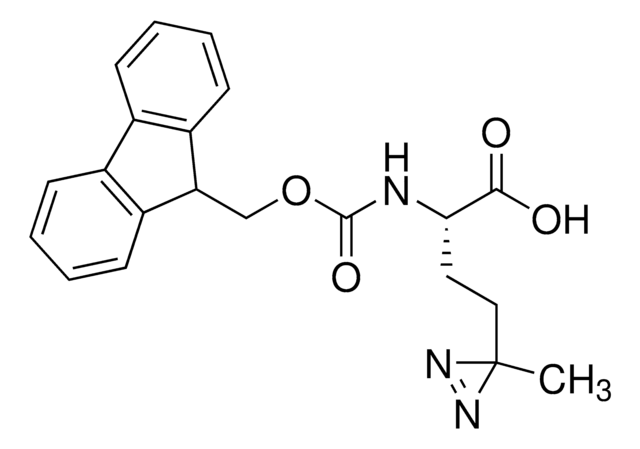
907340
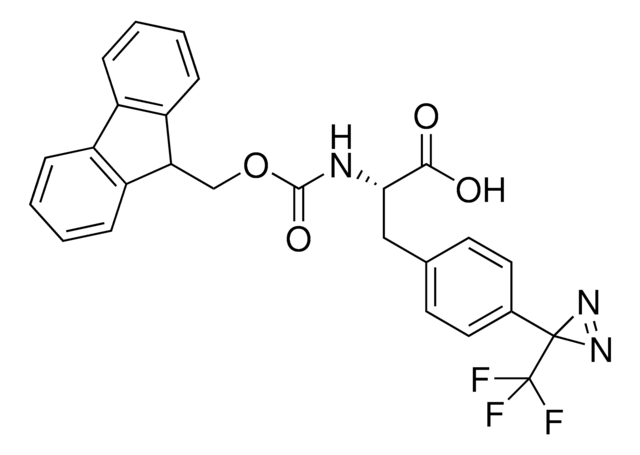
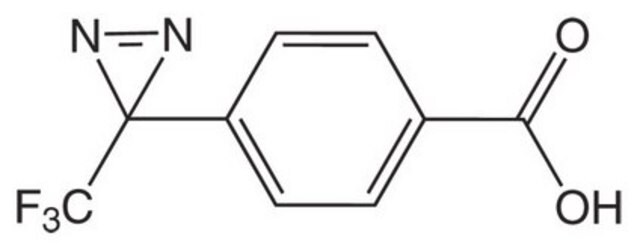
H-L-Photo-Phe-OH
≥98%
Synonyme(s) :
(S)-2-Amino-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, 4-(Trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, H-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Produits recommandés
Pureté
≥98%
Forme
powder
Capacité de réaction
reaction type: solution phase peptide synthesis
Disponibilité
available only in USA
Application(s)
peptide synthesis
Température de stockage
−20°C
InChI
1S/C11H10F3N3O2/c12-11(13,14)10(16-17-10)7-3-1-6(2-4-7)5-8(15)9(18)19/h1-4,8H,5,15H2,(H,18,19)
Clé InChI
HRGXDARRSCSGOG-UHFFFAOYSA-N
Catégories apparentées
Application
photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An Fmoc-protected version is also available as 907294.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Autres remarques
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
A genetically encoded diazirine photo-crosslinker in Escherichia coli
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Self-react. C
Code de la classe de stockage
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique