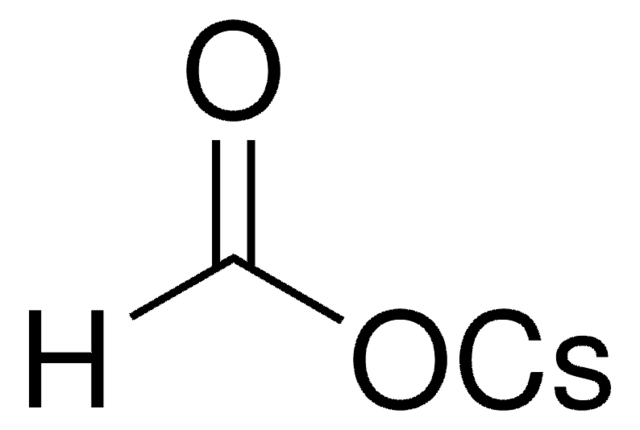
901111
Phenox O-PC™ A0202
New Iridium, ≥97%
Synonyme(s) :
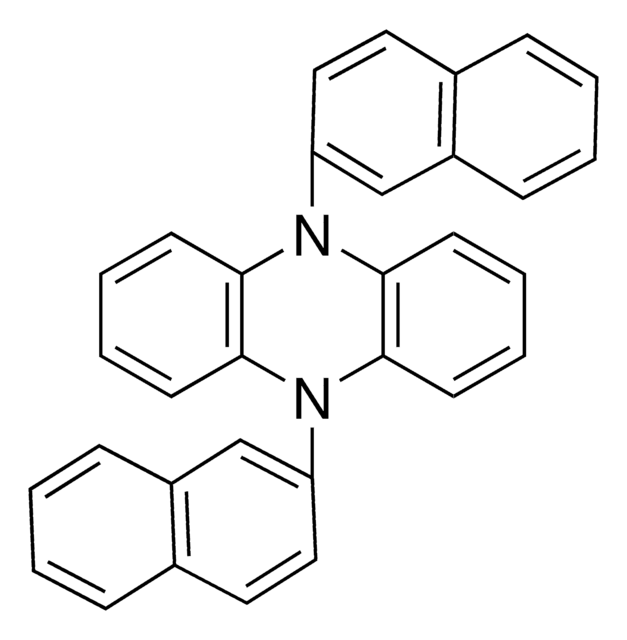
3,7-Di([1,1′-biphenyl]-4-yl)-10-(naphthalen-1-yl)-10H-phenoxazine, Miyake organophotoredox catalyst, 3,7-Di(4-biphenyl) 1-naphthalene-10-phenoxazine, PhenO_1Naph_Biph
About This Item
Produits recommandés
Niveau de qualité
Essai
≥97%
Forme
powder or crystals
Pertinence de la réaction
reagent type: catalyst
reaction type: Photocatalysis
Pf
171 °C
Activation du photocatalyseur
400 nm
Chaîne SMILES
C1(C=CC(C2=CC=C(C3=CC=CC=C3)C=C2)=C4)=C4OC(C=C(C5=CC=C(C6=CC=CC=C6)C=C5)C=C7)=C7N1C8=C(C=CC=C9)C9=CC=C8
InChI
1S/C46H31NO/c1-3-10-32(11-4-1)34-18-22-36(23-19-34)39-26-28-43-45(30-39)48-46-31-40(37-24-20-35(21-25-37)33-12-5-2-6-13-33)27-29-44(46)47(43)42-17-9-15-38-14-7-8-16-41(38)42/h1-31H
Clé InChI
IGGSSEOAGCUGDJ-UHFFFAOYSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Autres remarques
Organocatalyzed Atom Transfer Radical Polymerization Driven by Visible Light
Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts
Intramolecular Charge Transfer and Ion Pairing in N,N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
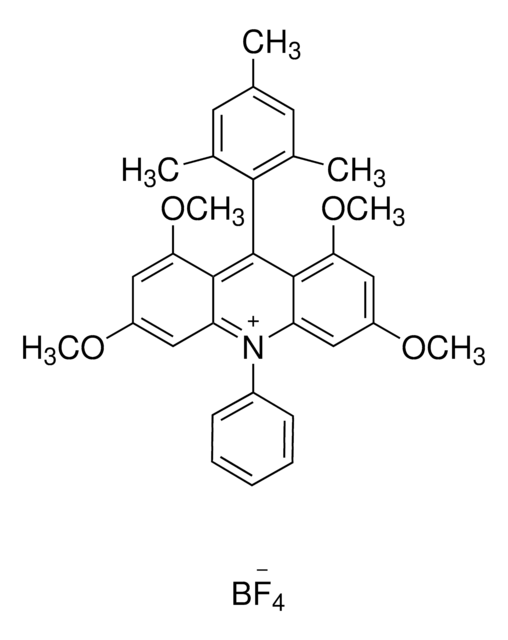
Les clients ont également consulté
Articles
Photoredox catalysis is a powerful synthetic methodology to form challenging covalent bonds using light irradiation. It is effective for light-driven polymer and small molecule synthesis.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)





![Tris[2-phenylpyridinato-C2,N]iridium(III) sublimed grade](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)