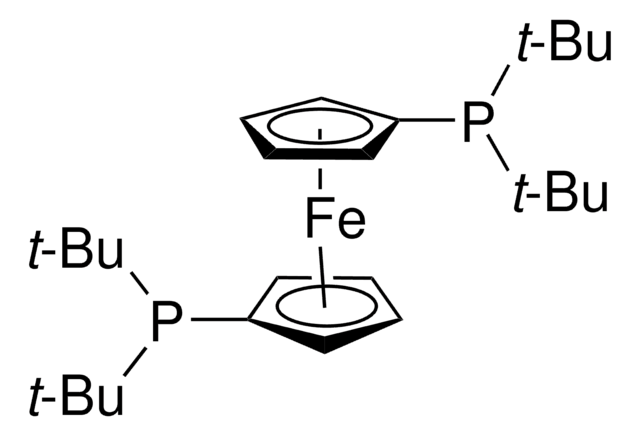
701602
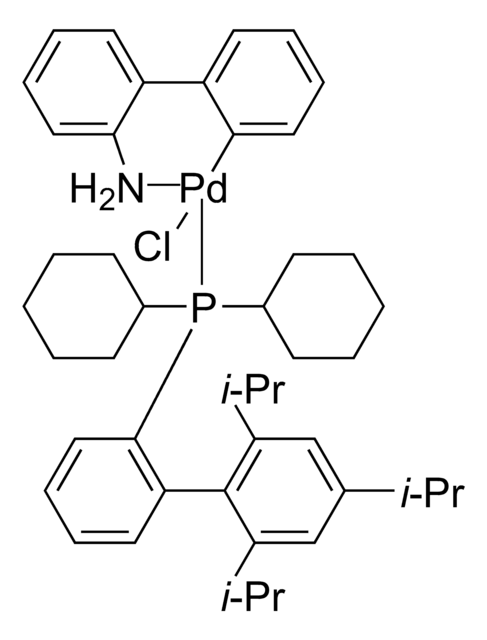
1,1′-Bis-(di-tert.-butylphosphino-)ferrocen-palladiumdichlorid
98%
Synonyme(s) :
PdCl2(dtbpf)
About This Item
Produits recommandés
Niveau de qualité
Essai
98%
Forme
powder
Pertinence de la réaction
core: palladium
reagent type: catalyst
Capacité de réaction
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
Caractéristiques du produit alternatif plus écologique
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Pf
203-208 °C
Autre catégorie plus écologique
, Aligned
Température de stockage
−20°C
Chaîne SMILES
[Fe].Cl[Pd]Cl.CC(C)(C)P([C]1[CH][CH][CH][CH]1)C(C)(C)C.CC(C)(C)P([C]2[CH][CH][CH][CH]2)C(C)(C)C
InChI
1S/2C13H22P.2ClH.Fe.Pd/c2*1-12(2,3)14(13(4,5)6)11-9-7-8-10-11;;;;/h2*7-10H,1-6H3;2*1H;;/q;;;;;+2/p-2
Clé InChI
JQZFOBWXNREQLO-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
PdCl2(dtbpf) is an air-stable cross-coupling catalyst used in the Suzuki coupling of various aryl chlorides.
Application
It is also employed as catalyst for greener Suzuki cross-coupling in TPGS-750-M.
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Protocoles
TPGS-750-M, a second generation surfactant, may be used for Suzuki-Miyaura Reactions in Water at Room Temperature
Global Trade Item Number
| Référence | GTIN |
|---|---|
| 701602-250MG | 4061833565100 |
| 701602-1G | 4061826700914 |
| 701602-50G | 4061833411605 |
| 701602-5G | 4061833330906 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![[1,1′-bis(diphénylphosphino)ferrocène]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphénylphosphino)ferrocène]dichloropalladium(II), complexe avec le dichlorométhane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)