377104
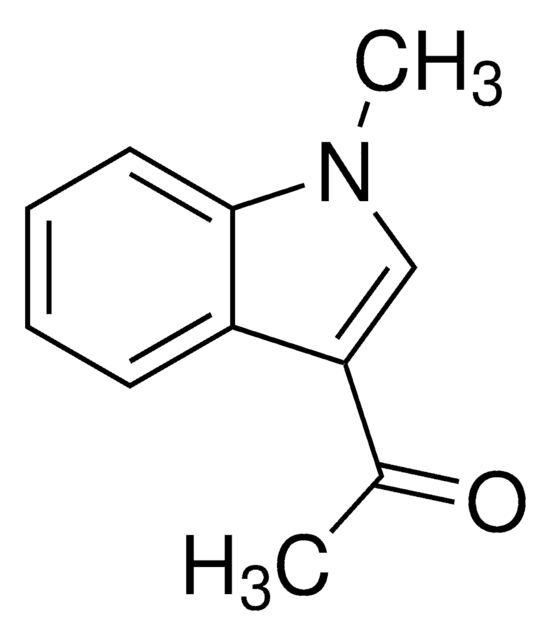
1-Acetylindole
98%
Synonyme(s) :
NSC 521758
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C10H9NO
Numéro CAS:
Poids moléculaire :
159.18
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
98%
Forme
liquid
Indice de réfraction
n20/D 1.607 (lit.)
Point d'ébullition
123-125 °C/8 mmHg (lit.)
Densité
1.387 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
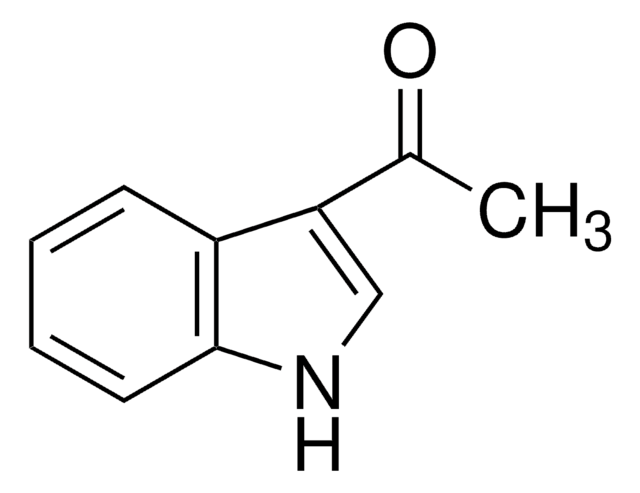
Chaîne SMILES
CC(=O)n1ccc2ccccc12
InChI
1S/C10H9NO/c1-8(12)11-7-6-9-4-2-3-5-10(9)11/h2-7H,1H3
Clé InChI
UUCUQJHYUPXDHN-UHFFFAOYSA-N
Description générale
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole has been carried out using density functional (DFT/B3LYP) method. Regioselective acylations of 1-acetylindole (N-acetylindole) under Friedel-Crafts reaction has been reported. Reaction of 1-acetylindole with manganese(III) acetate in the presence of malonic acid, is reported to afford 4-acetyl-3,3a,4,8b-tetrahydro-2H-furo[3,2-b]indol-2-one.
Application
1-Acetylindole may be used in the stereocontrolled synthesis of (±)-geissoschizine. It may be used in the preparation of (1-acetyl-κO-indolyl-κC2)tetracarbonylmanganese, via a standard cyclomanganation procedure.
Reactant for preparation of:
Reactant for:
- Antimycobacterial agents
- Cyclin-dependent kinase (CDK2) inhibitors
Reactant for:
- C3-C3 oxidative cross-coupling reactions
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Regioselective acylations at the 2 and 6 position of N-acetylindole.
Cruz R, et al.
Tetrahedron Letters, 42(8), 1467-1469 (2001)
Mangenese (III) acetate oxidation of 1-acetylindole derivatives.
Izumi T, et al.
Journal of Heterocyclic Chemistry, 30(4), 1133-1136 (1993)
Vikas K Shukla et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 133, 626-638 (2014-07-06)
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole were carried out using density functional (DFT/B3LYP) method with 6-311++G(d,p) basis set. The FT-IR and FT-Raman spectra were recorded in the condensed state. The fundamental vibrational
Synthesis and alkyne-coupling chemistry of cyclomanganated 1-and 3-acetylindoles, 3-formylindole and analogues.
Depree GJ, et al.
Journal of Organometallic Chemistry, 691(4), 667-679 (2006)
A concise, stereoselective synthesis of (?)-geissoschizine.
Bennasar M, et al.
Tetrahedron Letters, 37(50), 9105-9106 (1996)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique