375349
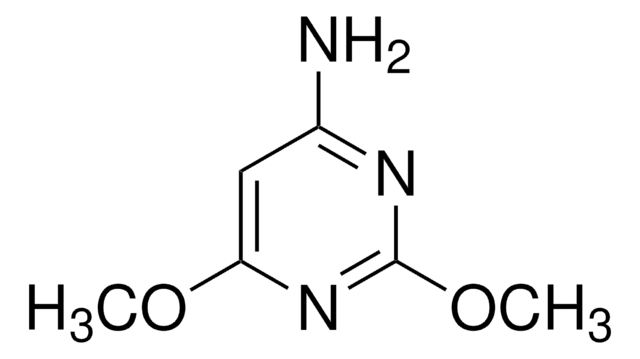
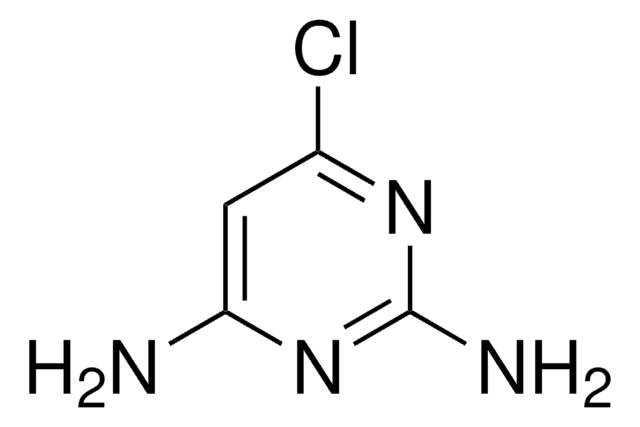
2-Amino-4,6-dimethoxypyrimidine
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C6H9N3O2
Numéro CAS:
Poids moléculaire :
155.15
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
98%
Pf
94-96 °C (lit.)
Chaîne SMILES
COc1cc(OC)nc(N)n1
InChI
1S/C6H9N3O2/c1-10-4-3-5(11-2)9-6(7)8-4/h3H,1-2H3,(H2,7,8,9)
Clé InChI
LVFRCHIUUKWBLR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
2-Amino-4,6-dimethoxypyrimidine is identified as degradation product of sulfosulphuron in agricultural soil by ESI LC-MS/MS analysis. It was isolated as metabolite during the biodegradation of bensulphuron-methyl by Penicillium pinophilum by liquid chromatography-mass spectrometry. The FTIR and FT-Raman spectra of 2-amino-4,6-dimethoxypyrimidine has been reported. Hydrogen bonding in the molecules of 2-amino-4,6-dimethoxypyrimidine has been investigated.
Application
2-Amino-4,6-dimethoxypyrimidine is suitable for use in the preparation of cocrystal 2-amino-4,6-dimethoxypyrimidine-anthranilic acid (1/1).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
John N Low et al.
Acta crystallographica. Section C, Crystal structure communications, 58(Pt 5), o289-o294 (2002-05-02)
Molecules of 2-amino-4,6-dimethoxypyrimidine, C(6)H(9)N(3)O(2), (I), are linked by two N-H.N hydrogen bonds [H.N 2.23 and 2.50 A, N.N 3.106 (2) and 3.261 (2) A, and N-H.N 171 and 145 degrees ] into a chain of fused rings, where alternate rings
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 65(5), 1186-1196 (2006-07-11)
The FTIR and FT-Raman spectra of 2-amino-4,6-dimethoxypyrimidine (2A46DMP) has been recorded in the region 4000-400 cm(-1) and 3500-100 cm(-1), respectively. The optimized geometry, frequency and intensity of the vibrational bands of 2A46DMP were obtained by the ab initio and DFT
U Yadav et al.
Letters in applied microbiology, 59(5), 479-486 (2014-07-22)
Sulfosulphuron-degrading fungus was isolated by enrichment technique from the sulfosulphuron-contaminated soil of wheat rhizosphere. To assess the biodegradation potential of isolated Trichoderma sp., minimal potato dextrose agar broth with different levels of sulfosulphuron (up to 2 g l(-1) ) was evaluated in
Caitlin Rering et al.
Journal of agricultural and food chemistry, 65(15), 3103-3108 (2017-04-04)
Imazosulfuron, a sulfonylurea herbicide used in rice cultivation, has been shown to undergo photodegradation in water, but neither the photochemical mechanism nor the role of indirect photolysis is known. The purpose of this study was to investigate the underlying processes
Kaliyaperumal Thanigaimani et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 1), o107-o108 (2007-01-01)
In the title cocrystal, C(6)H(9)N(3)O(2)·C(7)H(7)NO(2), the asymmetric unit contains two crystallographically independent 2-amino-4,6-dimeth-oxy pyrimidine-anthranilic acid adducts. The 2-amino-4,6-dimeth-oxy pyrimidine mol-ecules inter-act with the carboxylic group of the respective anthranilic acid mol-ecules through N-H⋯O and O-H⋯N hydrogen bonds, forming a cyclic
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique