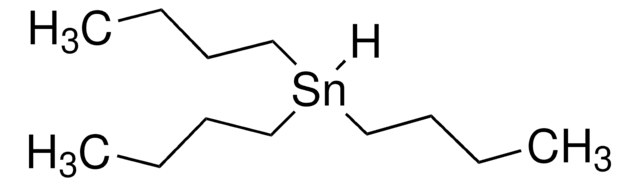
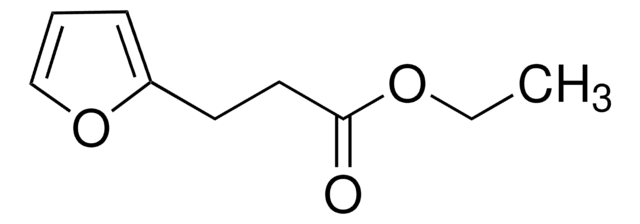
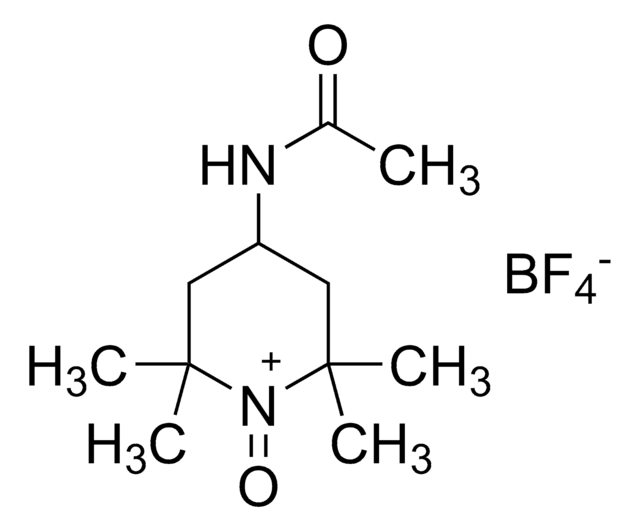
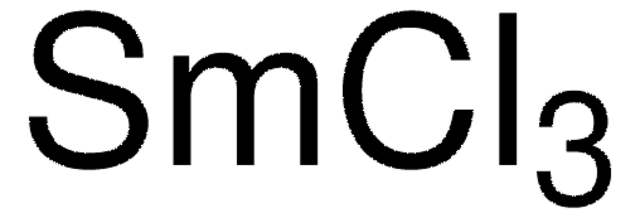347116
Samarium(II) iodide solution
0.1 M in THF, contains samarium chips as stabilizer
Synonyme(s) :
Samarium diiodide
About This Item
Produits recommandés
Forme
liquid
Niveau de qualité
Contient
samarium chips as stabilizer
Pertinence de la réaction
core: samarium
reagent type: catalyst
reaction type: Reductions
reagent type: reductant
Concentration
0.1 M in THF
Densité
0.922 g/mL at 25 °C
Température de stockage
2-8°C
Chaîne SMILES
I[Sm]I
InChI
1S/2HI.Sm/h2*1H;/q;;+2/p-2
Clé InChI
UAWABSHMGXMCRK-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Benzannulated pyrrolizidines and indolizidines by SmI2-induced cyclizations of indole derivatives.
- Chiral 4-substituted 2-oxazolidinones and 5,5-disubstituted oxazolidinones through asymmetric Reformatsky type reaction.
- γ-Aminoalkyl substituted γ-butyrolactones via ketyl-alkene coupling reaction.
It can also be used in:
- Reduction of conjugated double and triple bonds into alkenes using SmI2/H2O/amine mixtures.
- Conversion of β-hydroxyketones into 1,3-diols by SmI2/H2O/Et3N.
- Selective reduction of 6-membered lactones to the corresponding diols/triols using SmI2-H2O reagent system.
SmI2 is an effective single-electron reducing agent for the promotion of ketone-olefin, ketyl aryl cyclizations, and pinacol coupling reactions under mild conditions. Often both intramolecular and intermolecular couplings proceed in a highly stereoselective fashion. It is also used in the synthesis of new heteroleptic samarium aryloxide/cyclopentadienide complexes.
Conditionnement
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3 - Water-react 3
Organes cibles
Central nervous system, Respiratory system
Risques supp
Code de la classe de stockage
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
-2.2 °F - closed cup - Solvent
Point d'éclair (°C)
-19 °C - closed cup - Solvent
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![Tris[N,N-bis(trimethylsilyl)amide]samarium(III) 98%](/deepweb/assets/sigmaaldrich/product/structures/285/605/c4a36589-b92a-45c3-83a9-806ca49f392d/640/c4a36589-b92a-45c3-83a9-806ca49f392d.png)