Cell Bank Manufacturing
Your master cell bank (MCB) is critical to therapeutic product development, in addition to supporting clinical development, manufacturing, and commercial supply of biologics. Get it right the first time by letting us handle your MCB production at our cGMP facilities in the U.S. and U.K., as well as production of your working cell bank (WCB) to ensure high quality and easy scale-up when it’s time to begin manufacturing.
We can produce cell banks of up to 1,000 vials at our facilities in our traditional or closed process. Our industry-leading production experience spans a wide range of cell lines:
|
|
|
Closed processing cell bank manufacturing
Closed processing is designed for your suspension cell lines. Our team grow your cells in a fully closed system assessed using a detailed and documented contamination control strategy. Choosing to produce your cell banks in a closed process reduces the risk of cross-contamination and allows for automation which reduces manual errors.
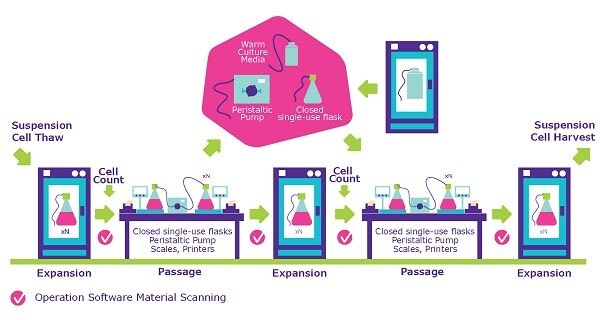
Closed processing limits the possibility of contaminants by closing the steps in the expansion process
Related Resources
- Webinar: Kick It Off Right: A Successful Cell Bank Manufacture
Watch this webinar to learn how to get the most out of your cell bank.
- Webinar: From Myth to Mastery: Unveiling the Secrets of Virus Bank Manufacturing
In this webinar, we will walk you through the GMP manufacturing steps using case studies and real-life examples.
- Infographic: Cell Line Preparation for Optimal Production
In this infographic, we show the 3 main steps for optimal cell line preparation that will give you peace of mind for your production process.
- Technical Note: Transition to Closed Processing Systems
Read this tech note to see how commonly banked cell types fared using traditional versus closed culture systems.
- Flyer: Confidence, Closed processing cell bank manufacturing
Discover how closed processing reduce the risk of cross-contamination in your cell bank manufacturing.
- Flyer: Cell Banking and Storage
We ensure your cell lines are produced and maintained for optimal production.
Request Information
To continue reading please sign in or create an account.
Don't Have An Account?