Built to Serve
Your breakthroughs. Your milestones. Built to serve you.
Millipore® Contract Testing, Development, and Manufacturing Organization (CTDMO) Services
With Millipore® CTDMO Services we provide a streamlined experience in a single partner to mitigate the burden of managing multiple vendors, supply chain needs, and complex priorities along the drug life cycle. We combine our expertise in development, manufacturing, contract testing and technologies from pre-clinical to commercial to accelerate our clients’ milestones and breakthroughs — as one experienced partner.
We leverage 30+ years of global success working with drug development, material science, and process technology aligned with our supply network to accelerate our clients’ programs.
We are the industry’s experienced choice — built to serve.
Experience the Advantage with Integrated Testing
Our CTDMO services provide a unified and simplified experience covering the entire value chain, giving clients one trusted partner along the drug development journey. Our contract testing services are integrated into the modality services to further streamline the development path. Our expertise, speed, and agility are what make the difference for our clients’ breakthrough innovations.
The Value We Provide
Known for our deep expertise & broad portfolio – with standard-setting innovations & template development to push the industry forward.
Flexibility through manufacturing and testing capacity – making significant investment in our capabilities and capacity to scale with the industry’s complex supply chain needs.
Decades of experience to inform and navigate clients’ pathways to approval. Our global regulatory know-how ensures high quality standards and regulatory compliance.
Focused Leadership Across Modalities

ADC & Bioconjugation
Antibody-drug conjugates (ADC) require a combination of precise design and specialized expertise — all delivered at speed. With 15+ years of contract development and manufacturing experience, we are recognized for leading expertise in the conjugation of drug linkers to monoclonal antibodies (mAbs) with facilities specialized for handling highly potent compounds.

mAb & r-Protein
Whether the biomolecule is a monoclonal antibody, bispecific antibody, ADC, or a fusion protein, we help build a robust, scalable process — ensuring success now and into the future. With our large-scale GMP facilities and more than 260 biologics, molecules are in experienced hands with a proven scale-up success rate.

mRNA Manufacturing & LNP Formulation
The mRNA landscape is rapidly changing, and clients need the latest novel technology and new therapeutic approaches to bring molecules to life. With the most comprehensive mRNA value chain in the market and integrated services, we successfully support clients from mRNA through lipids to LNP Formulation.

Small Molecule
Small in name, large in patient impact. We offer a true consultative partner approach to a broad spectrum of small molecule modalities. From APIs to HPAPIs, PEGs to ADC linker-payloads, targeted protein degradation to novel format conjugates, our decades of experience reduces complexity and minimizes risk in the race to commercialization.

Viral Vector
Our expertise with adeno-associated virus, lentivirus, adenovirus, and other vectors streamline the development and manufacturing of gene or cell therapy. Put trust in a partner with more than 25 years of relentless innovation of viral vector commercialization – setting the standard through the latest technologies and processes at speed. Whether clients are in pre-clinical phase or nearing commercialization, we have the resources, capabilities, and knowledge to advance current and future therapies.
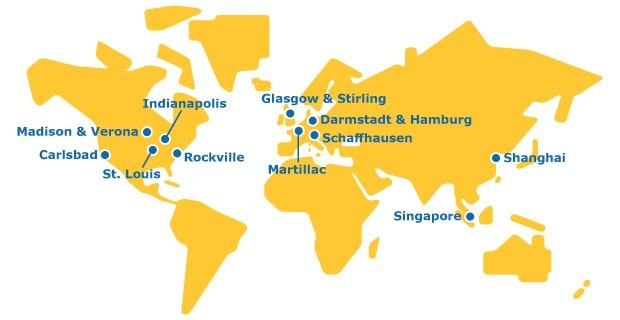
The Global Footprint of Our CTDMO Manufacturing Sites
We are a single organization with a global network to deliver CTDMO services across all stages of the molecule value chain.
Europe

Commercialization for large scale API production and portfolio lipids. Continued investment to expand our global network of API production facilities offers immediate development and manufacturing capacity.

Our biosafety testing service site houses our cell bank manufacturing, biorepository, cell line characterization, and drug substance and drug product release testing services.

Our mRNA manufacturing site for our integrated CDMO capabilities includes the development and manufacturing of custom mRNAs.

Biopharma development and manufacturing facility for production of mAbs with 25+ years of GMP experience. Including pilot, and GMP capabilities.

Development, manufacturing and launch site for APIs, functionalized polyethylene glycols (PEGs), and lipids.

Our site features viral clearance study suites. Our Provise™ Clearance Service deploys a team of highly trained & experienced process scientists and study directors on your behalf, to perform all your required process steps at our state-of-the-art Stirling facility.
Asia

Our Shanghai site is home to our China Biologics Testing Center, which houses viral clearance suites. This enables clients to locally conduct viral clearance studies from pre-clinical development to commercialization for in China and for global export.

Biosafety testing service site conducting cell line characterization, viral clearance, and drug substance and drug product release testing for our integrated CTDMO.
To continue reading please sign in or create an account.
Don't Have An Account?




